Unconventional secretion of Acb1 is mediated by autophagosomes - PubMed (original) (raw)
Unconventional secretion of Acb1 is mediated by autophagosomes
Juan M Duran et al. J Cell Biol. 2010.
Abstract
Starving Dictyostelium discoideum cells secrete AcbA, an acyl coenzyme A-binding protein (ACBP) that lacks a conventional signal sequence for entering the endoplasmic reticulum (ER). Secretion of AcbA in D. discoideum requires the Golgi-associated protein GRASP. In this study, we report that starvation-induced secretion of Acb1, the Saccharomyces cerevisiae ACBP orthologue, also requires GRASP (Grh1). This highlights the conserved function of GRASP in unconventional secretion. Although genes required for ER to Golgi or Golgi to cell surface transport are not required for Acb1 secretion in yeast, this process involves autophagy genes and the plasma membrane t-SNARE, Sso1. Inhibiting transport to vacuoles does not affect Acb1 secretion. In sum, our experiments reveal a unique secretory pathway where autophagosomes containing Acb1 evade fusion with the vacuole to prevent cargo degradation. We propose that these autophagosome intermediates fuse with recycling endosomes instead to form multivesicular body carriers that then fuse with the plasma membrane to release cargo.
Figures
Figure 1.
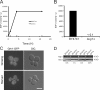
Yeast release SDF-2–like material. (A) Amino acid sequence of the AcbA homologues in S.cerevisiae, D. discoideum, and Homo sapiens are aligned. (B) The assay for the activity of Acb1. Yeast cells were starved for the time indicated. The cells were separated from the medium by low speed centrifugation and lysed. The lysates were used for detection of the intracellular levels of Acb1 and the control protein 3-phosphoglycerate kinase (Pgk1). The medium was processed to concentrate Acb1 activity, which was then tested for its ability to sporulate D. discoideum KP cells and quantified. (C) Developed KP cells were incubated with buffer in which yeast had been starved for 4 h and the number of spores counted 1 h later. (D) Protein levels were assessed by Western blotting the yeast cell lysates with an anti-Pgk1p (3-phosphoglycerate kinase) antibody, which is a cytosolic protein used as a control, and anti-Acb1. Anti-Flag antibody shows expression of the tagged version of Acb1-Flag. (E) Time course of secretion. Yeast cells were starved, and samples were taken every 30 min up to 4 h. The buffer was assayed for SDF-2–like activity. The data show an average of three experiments.
Figure 2.
GRASP is required for Acb1 secretion. (A) Wild-type and grh1Δ yeast were incubated in starvation buffer for 0, 1, 4, and 16 h, and the supernatant was tested in the bioassay. (B) Wild-type and bug1Δ yeast were starved for 4 h and the supernatant analyzed in the bioassay. (C) A wild-type strain expressing a GFP-tagged version of Grh1 was starved for 4 h and visualized by fluorescence microscopy. Starvation does not change the punctate pattern of Grh1 localization. Bar, 5 µm. (D) Cells were treated as in A, and the cell lysates were analyzed to monitor the respective protein levels. Deletion of Grh1 does not affect the intracellular levels of 3-phosphoglycerate kinase or Acb1. The data show an average of three experiments.
Figure 3.
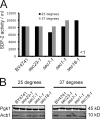
Conventional secretory pathway is not required for Acb1 secretion. (A) Yeast were grown in rich medium at 25°C, washed, and starved for 4 h in acetate buffer at the permissive (25°C) or restrictive temperature (37°C). SDF-2–like activity was determined in the bioassay. The data show an average of three experiments. (B) Cells from A were lysed and Western blotted with anti-Pgk1 and anti-Acb1 antibodies to monitor protein levels at the permissive and nonpermissive temperature.
Figure 4.
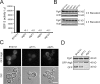
Autophagy genes are required for Acb1 secretion. (A) Yeast mutants deleted of atg genes 5, 7, 8, and 12 were incubated in starvation medium for 4 h. The buffer was tested in the bioassay. The data show an average of three experiments. (B) Cells described in A were Western blotted with anti-Acb1 antibody. Atg mutants do not show any change in the intracellular levels of Acb1. (C) Wild-type yeast, grh1Δ, and atg7Δ were transformed with a plasmid expressing GFP-Atg8 under the control of its endogenous promoter. The cells were starved as in A. Wild-type and grh1Δ strains show GFP fluorescence inside the vacuolar compartment. However, the atg7Δ strain lacked GFP fluorescence in the vacuole because of a defect in autophagy. Bar, 5 µm. (D) Wild-type, grh1Δ, and atg7Δ cells expressing GFP-Atg8 were starved for 4 h, and cell lysates were Western blotted with anti-GFP antibody to monitor the vacuolar proteolysis of GFP-Atg8. Wild type and grh1Δ, but not atg7Δ cells, showed a lower band with the apparent molecular weight of GFP alone caused by the proteolysis of GFP-Atg8 by vacuolar proteases (Shintani and Klionsky, 2004).
Figure 5.
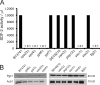
Early endosomal and MVB components are required for Acb1 secretion. (A) Wild-type and deletion mutants for the genes shown were starved for 4 h, and the SDF-2–like activity in the buffer was determined in the bioassay. The data show an average of three experiments. (B) Yeast from A was lysed, and equal amounts were Western blotted with anti-Pgk1 and anti-Acb1 antibody. Deletion mutants are compared with their wild-type isogenic strain (BY4741 or SEY6210). The intracellular levels of Acb1 are not affected in yeast mutants tested in this experiment.
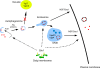
Figure 6.
The Acb1 secretion pathway. Cytosolic Acb1 is packaged into autophagosomes, which fuse with early endosomes. The early endosomes containing Acb1 either fuse directly with the cell surface or more likely mature into an MVB. The MVB fuses with the cell surface to release exosomes containing Acb1. Grh1 is required for the secretion of Acb1 and has to be membrane associated for its role in this pathway. However, the exact site of action for Grh1 in unconventional secretion remains unknown.
Comment in
- Unconventional secretion by autophagosome exocytosis.
Pfeffer SR. Pfeffer SR. J Cell Biol. 2010 Feb 22;188(4):451-2. doi: 10.1083/jcb.201001121. Epub 2010 Feb 15. J Cell Biol. 2010. PMID: 20156968 Free PMC article.
Similar articles
- Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion.
Bruns C, McCaffery JM, Curwin AJ, Duran JM, Malhotra V. Bruns C, et al. J Cell Biol. 2011 Dec 12;195(6):979-92. doi: 10.1083/jcb.201106098. Epub 2011 Dec 5. J Cell Biol. 2011. PMID: 22144692 Free PMC article. - Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation.
Manjithaya R, Anjard C, Loomis WF, Subramani S. Manjithaya R, et al. J Cell Biol. 2010 Feb 22;188(4):537-46. doi: 10.1083/jcb.200911149. Epub 2010 Feb 15. J Cell Biol. 2010. PMID: 20156962 Free PMC article. - Role of autophagy in unconventional protein secretion.
Manjithaya R, Subramani S. Manjithaya R, et al. Autophagy. 2010 Jul;6(5):650-1. doi: 10.4161/auto.6.5.12066. Epub 2010 Jul 1. Autophagy. 2010. PMID: 20473033 Free PMC article. - Unconventional protein secretion triggered by nutrient starvation.
Cruz-Garcia D, Malhotra V, Curwin AJ. Cruz-Garcia D, et al. Semin Cell Dev Biol. 2018 Nov;83:22-28. doi: 10.1016/j.semcdb.2018.02.021. Epub 2018 Feb 28. Semin Cell Dev Biol. 2018. PMID: 29486236 Review. - Life and Death of Fungal Transporters under the Challenge of Polarity.
Dimou S, Diallinas G. Dimou S, et al. Int J Mol Sci. 2020 Jul 29;21(15):5376. doi: 10.3390/ijms21155376. Int J Mol Sci. 2020. PMID: 32751072 Free PMC article. Review.
Cited by
- Lipopolysaccharide induction of autophagy is associated with enhanced bactericidal activity in Dictyostelium discoideum.
Pflaum K, Gerdes K, Yovo K, Callahan J, Snyder ML. Pflaum K, et al. Biochem Biophys Res Commun. 2012 Jun 8;422(3):417-22. doi: 10.1016/j.bbrc.2012.05.006. Epub 2012 May 7. Biochem Biophys Res Commun. 2012. PMID: 22575510 Free PMC article. - Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion.
Bruns C, McCaffery JM, Curwin AJ, Duran JM, Malhotra V. Bruns C, et al. J Cell Biol. 2011 Dec 12;195(6):979-92. doi: 10.1083/jcb.201106098. Epub 2011 Dec 5. J Cell Biol. 2011. PMID: 22144692 Free PMC article. - Genes for plant autophagy: functions and interactions.
Kim SH, Kwon C, Lee JH, Chung T. Kim SH, et al. Mol Cells. 2012 Nov;34(5):413-23. doi: 10.1007/s10059-012-0098-y. Epub 2012 Jul 6. Mol Cells. 2012. PMID: 22772908 Free PMC article. Review. - Non-canonical autophagy in aging and age-related diseases.
Kumar AV, Mills J. Kumar AV, et al. Front Cell Dev Biol. 2023 Feb 23;11:1137870. doi: 10.3389/fcell.2023.1137870. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36910139 Free PMC article. Review. - Unconventional secretion by autophagosome exocytosis.
Pfeffer SR. Pfeffer SR. J Cell Biol. 2010 Feb 22;188(4):451-2. doi: 10.1083/jcb.201001121. Epub 2010 Feb 15. J Cell Biol. 2010. PMID: 20156968 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases