Dynamic T cell migration program provides resident memory within intestinal epithelium - PubMed (original) (raw)
. 2010 Mar 15;207(3):553-64.
doi: 10.1084/jem.20090858. Epub 2010 Feb 15.
Daniel Choo, Vaiva Vezys, E John Wherry, Jaikumar Duraiswamy, Rama Akondy, Jun Wang, Kerry A Casey, Daniel L Barber, Kim S Kawamura, Kathryn A Fraser, Richard J Webby, Volker Brinkmann, Eugene C Butcher, Kenneth A Newell, Rafi Ahmed
Affiliations
- PMID: 20156972
- PMCID: PMC2839151
- DOI: 10.1084/jem.20090858
Dynamic T cell migration program provides resident memory within intestinal epithelium
David Masopust et al. J Exp Med. 2010.
Abstract
Migration to intestinal mucosa putatively depends on local activation because gastrointestinal lymphoid tissue induces expression of intestinal homing molecules, whereas skin-draining lymph nodes do not. This paradigm is difficult to reconcile with reports of intestinal T cell responses after alternative routes of immunization. We reconcile this discrepancy by demonstrating that activation within spleen results in intermediate induction of homing potential to the intestinal mucosa. We further demonstrate that memory T cells within small intestine epithelium do not routinely recirculate with memory T cells in other tissues, and we provide evidence that homing is similarly dynamic in humans after subcutaneous live yellow fever vaccine immunization. These data explain why systemic immunization routes induce local cell-mediated immunity within the intestine and indicate that this tissue must be seeded with memory T cell precursors shortly after activation.
Figures
Figure 1.
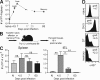
Only early effector CD8 T cells migrate to intestinal epithelium and express α4β7. (A) Dynamics of P14 response to LCMV. The day 37 time point represents 12 mice analyzed from days 31 to 50 after infection. (B) Experimental design consists of transferring P14 at different stages of differentiation and harvesting tissues the next day. (C) Numbers of naive (N), effector (isolated 4.5 or 7 d after infection), and memory (isolated 60 d after infection) cells isolated from recipient spleen and intestinal epithelium (IEL). ns, not significant, *, P < 0.05; **, P < 0.01, unpaired Student’s _t_ test. Error bars indicate SEM. (D) Virus-specific P14 CD8 T cells were analyzed for expression of α4β7. gmfi, geometric mean fluorescence intensity of α4β7 staining. All plots are gated on Thy1.1+ CD8+ lymphocytes and are representative of at least three independent experiments totaling >10 mice/time point.
Figure 2.
Antigen-dependent reexpression of α4β7 by spleen-derived transgenic and endogenous memory CD8 T cells upon infection with virus or bacteria. (A–D) Splenocytes isolated from P14 immune chimeras (>30 d after LCMV Arm infection) were transferred to naive recipients. (A and B) The next day, recipients were challenged with high-dose LCMV Arm or left unchallenged. (A) α4β7 expression was monitored in blood among donor P14 (Thy1.1+/gp33 tetramer+), nontransgenic gp33-specific cells (Thy1.1−/gp33 tetramer+), and CD44lo (naive) CD8 T cells. Representative flow cytometry data are shown. (B) Change in GMFI of α4β7 expression relative to α4β7 GMFI of memory P14 transferred to unchallenged mice that were analyzed on the same day. (C and D) As in A and B, except mice were challenged with LCMV Cl−13 (C) or LM-gp33 (D), or the noncognate antigen bearing inflammation control LM-WT. (E) 19 d after infection, small intestine IEL of these mice were examined for the presence of donor P14. (F) Splenocytes from LCMV Arm–immune C57BL/6J mice (which did not contain P14) were transferred to naive CD45.1 recipients. Recipients were challenged the next day with LM-gp33, and α4β7 expression among CD45.1− gp33-tetramer+ CD8 T cells was monitored in blood. At least three mice were analyzed at each time point in each experiment. Error bars indicate SEM. One of two experiments with similar results is shown.
Figure 3.
Memory CD8 T cells do not retain α4β7 expression regardless of anatomical location or immunization route. Naive Thy1.1+ P14 were transferred to naive mice. (A and B) The next day, mice were infected intranasally with 500 pfu of recombinant influenza virus that expresses gp33. 5 (A) or 86 (B) d later, lymphocytes were isolated from the indicated tissues and stained with α-Thy1.1, CD8, and α4β7 or CD44. Plots are gated on CD8+ lymphocytes. (C and D) Alternatively, 200 µg DNA that expresses the glycoprotein of LCMV under control of the CMVie promoter was administered intramuscularly into both anterior tibialis muscles. (C) 9 and 12 d later, Thy1.1+ cells were examined for expression of α4β7 (gated on CD8+ Thy1.1+ lymphocytes). (D) 12 d after immunization, lymphocytes were isolated from spleen and IEL and stained with α-CD8α, Thy1.1, and CD44 antibodies. Plots are gated on CD8α+ lymphocytes. (E) Naive Thy1.1+ P14 were transferred to naive C57BL/6J mice. Control mice received normal drinking water, whereas treated mice were exposed to 2 µg/ml FTY720 in the drinking water ad libitum for the duration of the experiment. The next day, both groups of mice were immunized with LCMV, and α4β7 expression among Thy1.1+ P14 in spleen was compared among control mice (black line), FTY720-treated mice (dashed line), and CD44lo CD8 T cells isolated from spleens of naive mice (gray histogram). Plots are gated on Thy1.1+ CD8+ lymphocytes. All data are representative of two experiments with at least three mice per group in each experiment.
Figure 4.
Memory CD8 T cells do not retain α4β7 expression regardless of anatomical location or immunization route. (A and B) Expression of α4β7 and/or CCR9 by P14 isolated from various tissues 4.5, 7, or 60 d after i.p. LCMV infection (A) or by OT-I after oral LMova infection (B). All plots are gated on Thy1.1+ CD8+ lymphocytes. (C) 106 Thy1.1+ P14 isolated from spleen (red), iLN (blue), or mLN (green) 4.5 d after LCMV infection was transferred to naive mice. The next day, lymphocytes were harvested from recipient spleen and small intestinal epithelium, and the proportion of CD8+ lymphocytes that were Thy1.1+ was determined. Only p-values of <0.05 are shown. (D) Recipient mice received the entire single cell suspension derived from either one spleen or the complete cluster of mLN derived from one mouse isolated 4.5 d after LCMV infection. The next day, the proportion of CD8+ lymphocytes that were Thy1.1+ was determined. Error bars indicate SEM. All data are representative of at least two experiments with at least three mice per group in each experiment.
Figure 5.
Memory CD8 T cells in intestinal epithelium do not recirculate. Naive P14 cells were transferred to naive C57BL/6J mice, and recipients were infected with LCMV. 90 d later, 2 µg/ml FTY720 was dissolved in drinking water (white bars) or mice were maintained on normal drinking water (black bars). (A and B) 2 (A) or 30 (B) d after FTY720 treatment, the number of LCMV-specific P14 memory CD8 T cells was determined in blood (PBL), iLNs, lung, or small intestinal epithelium (IEL). Data shown is one of five experiments with three mice per group with similar results. Immune mice were generated by transferring naive Thy1.1+ P14 into naive C57BL/6J mice and infecting recipients with LCMV. Error bars indicate SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001, unpaired Student’s t test. (C) 2 mo later, 7 cm of small intestine, along with associated mesentery and mLN, were transplanted from naive mice into immune mice. (D) 42 d after transplantation, lymphocytes were isolated from host spleen, blood (PBL), mLN, and intestinal epithelium (IEL), as well as donor mLN and IEL. The presence of host memory P14 was determined in each tissue by Thy1.1 staining and flow cytometry. All plots are gated on CD8+ lymphocytes and are representative of one of a total of three mice examined in two independent experiments. NA, not applicable.
Figure 6.
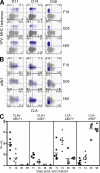
Primary human CD8 T cell response to s.c. yellow fever vaccine results in only short-term expression of α4β7 and CLA. Blood was isolated from HLA-A2–positive volunteers 11, 14, 30, and 90 d after s.c. vaccination with YFV-17D. (A) Expression of CLA versus staining with MHC class I tetramers that recognize YFV-specific CD8 T cells from three representative patients. (B) CLA versus α4β7 expression. HLA2-YFV tetramer+ cells are blue and HLA2-YFV tetramer− cells are gray. Numbers indicate percentage of tetramer+ cells in each quadrant. All plots are gated on CD3+ CD8+ lymphocytes. (C) Summary of CLA and α4β7 expression among YFV tetramer+ cells. Longitudinal analysis is shown, although some patients were not examined on days 30 and 90. Horizontal bars show the mean. Day 11, n = 7; day 14, n = 7; day 30, n = 4; day 90, n = 6.
Similar articles
- Intestinal and splenic T cell responses to enteric Listeria monocytogenes infection: distinct repertoires of responding CD8 T lymphocytes.
Huleatt JW, Pilip I, Kerksiek K, Pamer EG. Huleatt JW, et al. J Immunol. 2001 Mar 15;166(6):4065-73. doi: 10.4049/jimmunol.166.6.4065. J Immunol. 2001. PMID: 11238655 - Circulating memory CD8+ T cells are limited in forming CD103+ tissue-resident memory T cells at mucosal sites after reinfection.
Behr FM, Beumer-Chuwonpad A, Kragten NAM, Wesselink TH, Stark R, van Gisbergen KPJM. Behr FM, et al. Eur J Immunol. 2021 Jan;51(1):151-166. doi: 10.1002/eji.202048737. Epub 2020 Aug 31. Eur J Immunol. 2021. PMID: 32762051 - T-cell homing to the gut mucosa: general concepts and methodological considerations.
De Calisto J, Villablanca EJ, Wang S, Bono MR, Rosemblatt M, Mora JR. De Calisto J, et al. Methods Mol Biol. 2012;757:411-34. doi: 10.1007/978-1-61779-166-6_24. Methods Mol Biol. 2012. PMID: 21909925 - Lymphocyte traffic to mucosa-associated lymphatic tissues.
Jalkanen S. Jalkanen S. Immunol Res. 1991;10(3-4):268-70. doi: 10.1007/BF02919705. Immunol Res. 1991. PMID: 1955753 Review. No abstract available. - T lymphocyte trafficking: molecules and mechanisms.
Fu H, Wang A, Mauro C, Marelli-Berg F. Fu H, et al. Front Biosci (Landmark Ed). 2013 Jan 1;18(2):422-40. doi: 10.2741/4111. Front Biosci (Landmark Ed). 2013. PMID: 23276933 Review.
Cited by
- Location, location, location: tissue-specific regulation of immune responses.
Hu W, Pasare C. Hu W, et al. J Leukoc Biol. 2013 Sep;94(3):409-21. doi: 10.1189/jlb.0413207. Epub 2013 Jul 3. J Leukoc Biol. 2013. PMID: 23825388 Free PMC article. Review. - The Characterization of Varicella Zoster Virus-Specific T Cells in Skin and Blood during Aging.
Vukmanovic-Stejic M, Sandhu D, Seidel JA, Patel N, Sobande TO, Agius E, Jackson SE, Fuentes-Duculan J, Suárez-Fariñas M, Mabbott NA, Lacy KE, Ogg G, Nestle FO, Krueger JG, Rustin MHA, Akbar AN. Vukmanovic-Stejic M, et al. J Invest Dermatol. 2015 Jul;135(7):1752-1762. doi: 10.1038/jid.2015.63. Epub 2015 Mar 3. J Invest Dermatol. 2015. PMID: 25734814 Free PMC article. - Old and new: recent innovations in vaccine biology and skin T cells.
Kupper TS. Kupper TS. J Invest Dermatol. 2012 Mar;132(3 Pt 2):829-34. doi: 10.1038/jid.2011.400. Epub 2012 Jan 12. J Invest Dermatol. 2012. PMID: 22237702 Free PMC article. Review. - Effector-like CD8⁺ T cells in the memory population mediate potent protective immunity.
Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Olson JA, et al. Immunity. 2013 Jun 27;38(6):1250-60. doi: 10.1016/j.immuni.2013.05.009. Epub 2013 Jun 6. Immunity. 2013. PMID: 23746652 Free PMC article. - Molecular mechanisms of CD8(+) T cell trafficking and localization.
Nolz JC. Nolz JC. Cell Mol Life Sci. 2015 Jul;72(13):2461-73. doi: 10.1007/s00018-015-1835-0. Epub 2015 Jan 11. Cell Mol Life Sci. 2015. PMID: 25577280 Free PMC article. Review.
References
- Belyakov I.M., Derby M.A., Ahlers J.D., Kelsall B.L., Earl P., Moss B., Strober W., Berzofsky J.A. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA. 95:1709–1714 10.1073/pnas.95.4.1709 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources