Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE - PubMed (original) (raw)
Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE
Marina O Ziehn et al. Lab Invest. 2010 May.
Abstract
Over half of multiple sclerosis (MS) patients experience cognitive deficits, including learning and memory dysfunction, and the mechanisms underlying these deficits remain poorly understood. Neuronal injury and synaptic loss have been shown to occur within the hippocampus in other neurodegenerative disease models, and these pathologies have been correlated with cognitive impairment. Whether hippocampal abnormalities occur in MS models is unknown. Using experimental autoimmune encephalomyelitis (EAE), we evaluated hippocampal neurodegeneration and inflammation during disease. Hippocampal pathology began early in EAE disease course, and included decreases in CA1 pyramidal layer volume, loss of inhibitory interneurons and increased cell death of neurons and glia. It is interesting to note that these effects occurred in the presence of chronic microglial activation, with a relative paucity of infiltrating blood-borne immune cells. Widespread diffuse demyelination occurred in the hippocampus, but there was no significant decrease in axonal density. Furthermore, there was a significant reduction in pre-synaptic puncta and synaptic protein expression within the hippocampus, as well as impaired performance on a hippocampal-dependent spatial learning task. Our results demonstrate that neurodegenerative changes occur in the hippocampus during autoimmune-mediated demyelinating disease. This work establishes a preclinical model for assessing treatments targeted toward preventing hippocampal neuropathology and dysfunction in MS.
Conflict of interest statement
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures
Figure 1
Clinical disease scores of mice with experimental autoimmune encephalomyelitis (EAE). EAE was experimentally induced in adult C57Bl/6 mice by myelin oligodendrocyte glycoprotein (MOG) immunization at days 0 and 7. Clinical signs began as early as day 7. Mice were sacrificed at early (day 13; n = 8) middle (day 35; n = 8) and late (day 55; n = 8) disease time points, in order to assess neuropathology in the hippocampus.
Figure 2
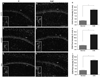
CA1 pathology in experimental autoimmune encephalomyelitis (EAE) includes decreases in pyramidal volume and numbers of GABA-ergic interneurons. Decreased pyramidal layer volume and interneuron loss occur in EAE mice at early (b), middle (f) and late (j) stages of disease, compared with gender and age-matched healthy mice (a, e and i). All panels are pseudo-colored confocal images taken at × 10 magnification. CA1 neurons (NeuN, Cy5-red), GABA-ergic interneurons (parvalbumin (PV), FitC-green) and cell nuclei (DAPI, blue) are depicted in images. Quantification of decreased pyramidal CA1 volume (c, g and k) and interneurons (d, h and l) at each time point of EAE shown to the right in graphs. *Denotes statistical significance compared with normal, (P<0.001); Student’s t test, n = 8 per condition, per time point. Subsequent experiments have confirmed these results. Scale bar, 20 µm.
Figure 3
Inflammation in the hippocampal CA1 region during experimental autoimmune encephalomyelitis (EAE). Microglial activation, identified by CD45+ labeling (Cy5-red), was measured in dorsal CA1 of early-, middle- and late-stage EAE mice, and compared with healthy age-matched normal mice. Normal mice and EAE mice are denoted by N and EAE. At all time points, EAE mice had significantly more CD45+ labeling than normal mice. CA1 seen by DAPI+ labeling (blue) of pyramidal cell layer. Images were taken at × 10 magnification, with small insets of normal and activated microglia, taken at × 40 magnification. Microglia in EAE have ramified morphology, indicative of inflammatory activation. Quantification of CD45+ pixel intensity (% area of immunoreactivity) shown in graphs c, f and i, with *indicating statistical significance, P<0.0001; n = 8 per condition, per time point. Scale bar, 20 µm.
Figure 4
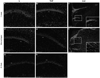
Hippocampal inflammation is not due to infiltrating T and B lymphocytes. At early time points, the hippocampus of normal and experimental autoimmune encephalomyelitis (EAE) mice did not stain for CD3 or CD19+ cells (Cy5-red), which label T cells and B cells, respectively (a, b, g, h). However, there was some macrophage/monocyte labeling seen in the CA1 of EAE mice (e) compared to normal mice (d). As a positive control for the ability to detect immune cell infiltration, thoracic spinal cord sections from EAE animals revealed both CD3+ and macrophage+ staining, (Cy5-red) (c and f). Sections were double-labeled for each antibody in conjunction with DAPI (blue), a nuclear DNA cell marker, and imaged at × 10 magnification using confocal microscopy. CA1 pyramidal layer is identified. Scale bar, 20 µm.
Figure 5
Widespread reduction of myelin in hippocampus during experimental autoimmune encephalomyelitis (EAE). Myelin basic protein (MBP, Cy5-red) staining was significantly reduced in the hippocampus of EAE mice (b), compared with normal control mice (a). Quantification of myelin pixel intensity depicted in graph c, where * indicates statistical significance, (P<0.001, Student’s _t_ test). Confocal images shown are taken at × 10 magnification from PLP-EGFP tissue sections, where PLP+ oligodendrocytes express EGFP (green), and cell nuclei are stained with DAPI (blue). EAE mice did not have significantly less PLP+ oligodendrocytes in the CA1 compared with normal mice (**a, b**). Tissue sections from Thy1-YFP (YFP-green) normal and EAE mice were stained with cell nuclei marker, DAPI (blue) to assess axonal area (**d**–**e**). To assess CA1 axons specifically, the stratum oriens was analyzed, (‘SO’ in panels). EAE mice did not have significantly different CA1 axonal area (**e**), compared with healthy normal control mice (**d**). Quantification is shown in graph **f**, where a Student’s t test yielded non-significant differences in CA1 axonal area, _P_>0.05. Scale bars, 20 µm.
Figure 6
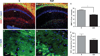
Increased cell death in the CA1 region of experimental autoimmune encephalomyelitis (EAE) mice. EAE mice had significantly more TUNEL+ cells in all layers of rostral CA1 (b and e), compared with normal control mice (a and d). TUNEL+ labeling (TMR-red) and cell nuclei (DAPI-blue) within CA1 are shown in × 10 confocal images (a and b) in normal and EAE mice. There were significantly more cells undergoing apoptosis in EAE mice compared with normal mice, quantification in graph (c). *Statistically significant, P<0.0001, Student’s unpaired t test, n = 8 per condition. d and e are × 40 magnified images representative of dashed squares in corresponding × 10 images. f and g are color split images of e. TUNEL+ cells are indeed DAPI+ (open arrow heads); however, not all DAPI+ cells are TUNEL+ (closed arrow heads). All scale bars are 20 µm.
Figure 7
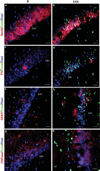
CA1 cell death occurs primarily in neurons and astrocytes, and occasionally in GABA-ergic interneurons and oligodendrocytes. Co-labeling experiments were used to identify the various cell types undergoing apoptosis in the CA1 of experimental autoimmune encephalomyelitis (EAE) mice. All panels depict × 40 magnified images of the dorsal CA1 of normal and EAE mice at early stages of disease. TUNEL+ cells are shown in pseudo-colored green in all panels, whereas each specific cell marker is red. Normal CA1 had sporadic TUNEL+ cells, and normal NeuN+ (a), PV+ (c), GFAP+ (e) and CNPase+ (g) labeling. The CA1 of EAE mice; however, showed significantly more TUNEL+ staining which co-labeled with NeuN (b), parvalbumin (PV) (d), GFAP (f) and relatively few CNPase co-labeling (e). All scale bars are 20 µm.
Figure 8
Synaptic loss in the CA1 region of experimental autoimmune encephalomyelitis (EAE) mice. Decreased synaptic protein, synapsin-1 (Syn-1; FitC-green) was observed in stratum radiatum, stratum pyramidale and stratum oriens of EAE mice (day 13, b) compared with healthy age-matched normal mice (a). Both immunoreactivity and quantity of synaptic puncta were significantly decreased in EAE hippocampus. Normal and EAE mice indicated by N and EAE in left and right columns. a and b depict dorsal CA1 cells (DAPI-blue) and Syn-1 immunoreactivity at high magnification, × 100, scale bar 20 µm. Graph (c) represents average number of Syn-1 immunoreactive puncta counted per stack, where EAE CA1 had approximately 40% decreased number of puncta compared with healthy control, *P<0.005, Student’s t test, n = 4 per condition.
Figure 9
Experimental autoimmune encephalomyelitis (EAE) causes a deficit in hippocampal-dependent spatial learning and memory at a later stage of disease. Spatial learning and memory was assessed in normal and EAE mice at two time points of disease (days 15 and 40). Graphs (a) and (c) show representative clinical disease courses for mice tested at an early time point (day 15), and mice tested at a later time point (day 40). On each test day, mice were each given three 300 s trials, in which they were to learn the location of a target hole in the Barnes Maze, and escape from noxious stimuli. Trials terminated at end of 300 s or when mouse entered target hole and escaped to target cage. Errors were defined as explorations into incorrect holes, and were counted and summed for each individual, then averaged by group to give total errors. At an early time point EAE and age-matched healthy control mice performed similarly in the Barnes Maze test, where both groups successfully committed fewer errors by the last trial of the day, graph (b). Later in disease, however, EAE mice had impaired performance, and committed significantly more errors during the last trial, than age-matched healthy controls, graph (d). Repeated-measures ANOVA yielded significant difference (*) after pair-wise comparisons, P<0.008; Bonferroni post-hoc analysis indicated significant difference between normal and EAE mice during last trial, P<0.01.
Similar articles
- Estriol preserves synaptic transmission in the hippocampus during autoimmune demyelinating disease.
Ziehn MO, Avedisian AA, Dervin SM, O'Dell TJ, Voskuhl RR. Ziehn MO, et al. Lab Invest. 2012 Aug;92(8):1234-45. doi: 10.1038/labinvest.2012.76. Epub 2012 Apr 23. Lab Invest. 2012. PMID: 22525427 Free PMC article. - Cognitive deficits in experimental autoimmune encephalomyelitis: neuroinflammation and synaptic degeneration.
Mandolesi G, Grasselli G, Musumeci G, Centonze D. Mandolesi G, et al. Neurol Sci. 2010 Nov;31(Suppl 2):S255-9. doi: 10.1007/s10072-010-0369-3. Neurol Sci. 2010. PMID: 20635112 Review. - Neurodegeneration and inflammation in hippocampus in experimental autoimmune encephalomyelitis induced in rats by one--time administration of encephalitogenic T cells.
Kurkowska-Jastrzębska I, Swiątkiewicz M, Zaremba M, Cudna A, Piechal A, Pyrzanowska J, Widy-Tyszkiewicz E, Członkowska A. Kurkowska-Jastrzębska I, et al. Neuroscience. 2013 Sep 17;248:690-8. doi: 10.1016/j.neuroscience.2013.06.025. Epub 2013 Jun 24. Neuroscience. 2013. PMID: 23806721 - Platelet-Activating Factor Receptors Mediate Excitatory Postsynaptic Hippocampal Injury in Experimental Autoimmune Encephalomyelitis.
Bellizzi MJ, Geathers JS, Allan KC, Gelbard HA. Bellizzi MJ, et al. J Neurosci. 2016 Jan 27;36(4):1336-46. doi: 10.1523/JNEUROSCI.1171-15.2016. J Neurosci. 2016. PMID: 26818520 Free PMC article. - Synaptic plasticity in multiple sclerosis and in experimental autoimmune encephalomyelitis.
Nisticò R, Mori F, Feligioni M, Nicoletti F, Centonze D. Nisticò R, et al. Philos Trans R Soc Lond B Biol Sci. 2013 Dec 2;369(1633):20130162. doi: 10.1098/rstb.2013.0162. Print 2014 Jan 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24298163 Free PMC article. Review.
Cited by
- Inhibition of Vascular Endothelial Growth Factor Receptor 2 Exacerbates Loss of Lower Motor Neurons and Axons during Experimental Autoimmune Encephalomyelitis.
Stanojlovic M, Pang X, Lin Y, Stone S, Cvetanovic M, Lin W. Stanojlovic M, et al. PLoS One. 2016 Jul 28;11(7):e0160158. doi: 10.1371/journal.pone.0160158. eCollection 2016. PLoS One. 2016. PMID: 27466819 Free PMC article. - Estriol preserves synaptic transmission in the hippocampus during autoimmune demyelinating disease.
Ziehn MO, Avedisian AA, Dervin SM, O'Dell TJ, Voskuhl RR. Ziehn MO, et al. Lab Invest. 2012 Aug;92(8):1234-45. doi: 10.1038/labinvest.2012.76. Epub 2012 Apr 23. Lab Invest. 2012. PMID: 22525427 Free PMC article. - Transcriptome Profiling in the Hippocampi of Mice with Experimental Autoimmune Encephalomyelitis.
Weerasinghe-Mudiyanselage PDE, Kang S, Kim JS, Kim JC, Kim SH, Wang H, Shin T, Moon C. Weerasinghe-Mudiyanselage PDE, et al. Int J Mol Sci. 2022 Nov 27;23(23):14829. doi: 10.3390/ijms232314829. Int J Mol Sci. 2022. PMID: 36499161 Free PMC article. - Therapeutic laquinimod treatment decreases inflammation, initiates axon remyelination, and improves motor deficit in a mouse model of multiple sclerosis.
Moore S, Khalaj AJ, Yoon J, Patel R, Hannsun G, Yoo T, Sasidhar M, Martinez-Torres L, Hayardeny L, Tiwari-Woodruff SK. Moore S, et al. Brain Behav. 2013 Nov;3(6):664-82. doi: 10.1002/brb3.174. Epub 2013 Sep 23. Brain Behav. 2013. PMID: 24363970 Free PMC article. - Neutrophil-selective deletion of Cxcr2 protects against CNS neurodegeneration in a mouse model of multiple sclerosis.
Khaw YM, Cunningham C, Tierney A, Sivaguru M, Inoue M. Khaw YM, et al. J Neuroinflammation. 2020 Feb 4;17(1):49. doi: 10.1186/s12974-020-1730-y. J Neuroinflammation. 2020. PMID: 32019585 Free PMC article.
References
- Vyse TJ, Todd JA. Genetic analysis of autoimmune disease. Cell. 1996;85:311–318. - PubMed
- Hemmer B, Nessler S, Zhou D. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat Clin Pract Neurol. 2006;2:201–211. - PubMed
- Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68(22) Supp 3:S22–S31. - PubMed
- Bö L, Geurts JJ, Mörk SJ, et al. Grey matter pathology in multiple sclerosis. Acta Neurol Scand. 2006;183 Suppl:48–50. - PubMed
- Cifelli A, Arridge M, Jezzard P, et al. Thalamic neurodegeneration in multiple sclerosis. Ann Neurol. 2002;52:650–653. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS045443-01/NS/NINDS NIH HHS/United States
- T32 HD007228/HD/NICHD NIH HHS/United States
- R01 NS045443-02/NS/NINDS NIH HHS/United States
- R01 NS045443-04/NS/NINDS NIH HHS/United States
- R01 NS045443-03/NS/NINDS NIH HHS/United States
- R01 NS45443/NS/NINDS NIH HHS/United States
- 5-T32-HD07228-26/HD/NICHD NIH HHS/United States
- T32 HD07228-26/HD/NICHD NIH HHS/United States
- R01 NS045443/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous