Orally ingested (13)C(2)-retinol is incorporated into hepatic retinyl esters in a nonhuman primate (Macaca mulatta) model of hypervitaminosis A - PubMed (original) (raw)
Orally ingested (13)C(2)-retinol is incorporated into hepatic retinyl esters in a nonhuman primate (Macaca mulatta) model of hypervitaminosis A
Anne L Escaron et al. Comp Med. 2010 Feb.
Abstract
The mechanism responsible for the metabolism of vitamin A during hypervitaminosis is largely unknown. This study investigated hepatic (13)C-retinol uptake in hypervitaminotic A rhesus monkeys. We hypothesized that individual retinyl esters would be enriched in (13)C after a physiologic dose of (13)C(2)-retinyl acetate, thus suggesting de novo in vivo hepatic retinol esterification. Male rhesus macaques (n = 16; 11.8 +/- 2.9 y) each received 3.5 micromol 14, 15-(13)C(2)-retinyl acetate. Blood was drawn at baseline and 5 h and 2, 4, 7, 14, 21, and 28 d after administration. Liver biopsies were collected 7 d before and 2 d after dose administration (n = 4) and at 7, 14, and 28 d after dose administration (n = 4 per time point). (13)C enrichments of retinol and retinyl esters HPLC-purified from liver samples were measured by using gas chromatography-combustion-isotope ratio mass spectrometry. (13)C enrichment of total vitamin A and individual retinyl esters were significantly greater 2 d after dose administration compared with baseline levels. In contrast, the concentration of isolated retinyl esters did not always increase 2 d after treatment. Given that the liver biopsy site differed between monkeys, these data suggest that the accumulation of hepatic retinyl esters is a dynamic process that is better represented by combining analytical techniques. This sensitive methodology can be used to characterize vitamin A trafficking after physiologic doses of (13)C-retinol. In this nonhuman primate model of hypervitaminosis A, hepatic retinyl esters continued to accumulate with high liver stores.
Figures
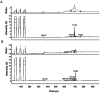
Figure 1.
(A) Mass chromatogram for labeled 13C2-retinol standard (50 ng/µL; 3% 13C2-retinol). (B) Mass chromatogram of retinol from saponified retinyl oleate and palmitate isolated from baseline rhesus monkey liver, indicating that the retinol saponified from hepatic retinyl esters at baseline has the same retention time as the labeled retinol standard. For both panels A and B, the top image indicates the ratio of CO2 molecules, whereas the bottom image indicates the signal strength (intensity of the peaks in volts). The dotted line in the top images represents the mass 45/44 trace—the variation of mass 45 (predominantly 13CO2) to mass 44 (12CO2). The solid line in the top panels represent the mass 46/44 trace —the variation of mass 46 (predominantly 12C18O16O) to mass 44 (12CO2). The first 3 peaks in both the top and bottom panel are the reference gas (CO2) pulses.
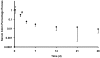
Figure 2.
Semilog plot of serum retinol enrichment over time in captive rhesus monkeys given an oral dose of 3.5 µmol 13C2-retinyl acetate. Serum retinol enrichment is shown as serum atom percentage excess (adjusted for baseline). Repeated measures using Tukey adjustment for multiple comparisons demonstrate that serum retinol enrichments at 5 h (*) and 2 d (#) after dose administration differ significantly (P < 0.05) from those at all other times. Values are presented as mean ± 1 SD (n = 16 per time point, except for 5 h and 28 d, for which n = 15).
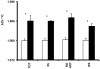
Figure 3.
Pre- and postdose At%13C for hepatic retinyl esters that were HPLC-purified and saponified to retinol: total retinyl esters (TOT), retinyl linoleate (RL), retinyl oleate and palmitate (RO + RP), and retinyl stearate (RS). Baseline (white bars) and postdose (black bars) values are given as mean ± 1 SD; values for baseline and day 2 after dose administration reflect the same 4 monkeys at each point. *, 1-way ANOVA by day revealed significant difference relative to baseline value (TOT, P = 0.0065; RL, P = 0.0003; RO + RP, P = 0.0022; and RS, P = 0.0027).
Similar articles
- Mathematical modeling of serum 13C-retinol in captive rhesus monkeys provides new insights on hypervitaminosis A.
Escaron AL, Green MH, Howe JA, Tanumihardjo SA. Escaron AL, et al. J Nutr. 2009 Oct;139(10):2000-6. doi: 10.3945/jn.109.111922. Epub 2009 Aug 26. J Nutr. 2009. PMID: 19710158 Free PMC article. - High provitamin A carotenoid serum concentrations, elevated retinyl esters, and saturated retinol-binding protein in Zambian preschool children are consistent with the presence of high liver vitamin A stores.
Mondloch S, Gannon BM, Davis CR, Chileshe J, Kaliwile C, Masi C, Rios-Avila L, Gregory JF 3rd, Tanumihardjo SA. Mondloch S, et al. Am J Clin Nutr. 2015 Aug;102(2):497-504. doi: 10.3945/ajcn.115.112383. Epub 2015 Jul 15. Am J Clin Nutr. 2015. PMID: 26178727 Free PMC article. Clinical Trial. - Plasma kinetics of vitamin A in humans after a single oral dose of [8,9,19-13C]retinyl palmitate.
Reinersdorff DV, Bush E, Liberato DJ. Reinersdorff DV, et al. J Lipid Res. 1996 Sep;37(9):1875-85. J Lipid Res. 1996. PMID: 8895053 - Comparisons among Equations Used for Retinol Isotope Dilution in the Assessment of Total Body Stores and Total Liver Reserves.
Gannon BM, Tanumihardjo SA. Gannon BM, et al. J Nutr. 2015 May;145(5):847-54. doi: 10.3945/jn.114.208132. Epub 2015 Mar 25. J Nutr. 2015. PMID: 25809683 Free PMC article. Review. - Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD).
Saeed A, Dullaart RPF, Schreuder TCMA, Blokzijl H, Faber KN. Saeed A, et al. Nutrients. 2017 Dec 29;10(1):29. doi: 10.3390/nu10010029. Nutrients. 2017. PMID: 29286303 Free PMC article. Review.
Cited by
- Retinol isotope dilution accurately predicts liver reserves in piglets but overestimates reserves in lactating sows.
Sheftel J, Surles RL, Tanumihardjo SA. Sheftel J, et al. Exp Biol Med (Maywood). 2019 May;244(7):579-587. doi: 10.1177/1535370219838785. Epub 2019 Mar 19. Exp Biol Med (Maywood). 2019. PMID: 30889962 Free PMC article. - Vitamin A status and body pool size of infants before and after consuming fortified home-based complementary foods.
Newton S, Owusu-Agyei S, Asante KP, Amoaful E, Mahama E, Tchum SK, Ali M, Adjei K, Davis CR, Tanumihardjo SA. Newton S, et al. Arch Public Health. 2016 Mar 7;74:10. doi: 10.1186/s13690-016-0121-4. eCollection 2016. Arch Public Health. 2016. PMID: 26955479 Free PMC article. - Vitamin A: biomarkers of nutrition for development.
Tanumihardjo SA. Tanumihardjo SA. Am J Clin Nutr. 2011 Aug;94(2):658S-65S. doi: 10.3945/ajcn.110.005777. Epub 2011 Jun 29. Am J Clin Nutr. 2011. PMID: 21715511 Free PMC article. Review.
References
- Amedee-Manesme O, Furr HC, Olson JA. 1984. The correlation between liver vitamin A concentrations in micro- (needle biopsy) and macrosamples of human liver specimens obtained at autopsy. Am J Clin Nutr 39:315–319 - PubMed
- Blaner WS, Hendriks HF, Brouwer A, de Leeuw AM, Knook DL, Goodman DS. 1985. Retinoids, retinoid-binding proteins, and retinyl palmitate hydrolase distributions in different types of rat liver cells. J Lipid Res 26:1241–1251 - PubMed
- Blaner WS, Olson JA. 1994. Retinol and retinoic acid metabolism, p 229–256 Sporn MB, Roberts AB, Goodman DS. The retinoids: biology, chemistry, and medicine, 2nd ed New York (NY): Raven Press
- Blomhoff R, Green MH, Green JB, Berg T, Norum KR. 1991. Vitamin A metabolism: new perspectives on absorption, transport, and storage. Physiol Rev 71:951–990 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR020141-01/RR/NCRR NIH HHS/United States
- 5P51 RR 000167/RR/NCRR NIH HHS/United States
- T32HP10010/PHS HHS/United States
- DK61973/DK/NIDDK NIH HHS/United States
- C06 RR020141/RR/NCRR NIH HHS/United States
- C06 RR015459/RR/NCRR NIH HHS/United States
- P51 RR000167/RR/NCRR NIH HHS/United States
- RR15459-01/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical