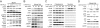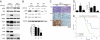ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS - PubMed (original) (raw)
. 2010 Mar 2;107(9):4153-8.
doi: 10.1073/pnas.0913860107. Epub 2010 Feb 16.
Sheng-Li Cai, Jinhee Kim, Adrian Nanez, Mustafa Sahin, Kirsteen H MacLean, Ken Inoki, Kun-Liang Guan, Jianjun Shen, Maria D Person, Donna Kusewitt, Gordon B Mills, Michael B Kastan, Cheryl Lyn Walker
Affiliations
- PMID: 20160076
- PMCID: PMC2840158
- DOI: 10.1073/pnas.0913860107
ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS
Angela Alexander et al. Proc Natl Acad Sci U S A. 2010.
Erratum in
- Proc Natl Acad Sci U S A. 2012 May 22;109(21):8352
Abstract
Ataxia-telangiectasia mutated (ATM) is a cellular damage sensor that coordinates the cell cycle with damage-response checkpoints and DNA repair to preserve genomic integrity. However, ATM also has been implicated in metabolic regulation, and ATM deficiency is associated with elevated reactive oxygen species (ROS). ROS has a central role in many physiological and pathophysiological processes including inflammation and chronic diseases such as atherosclerosis and cancer, underscoring the importance of cellular pathways involved in redox homeostasis. We have identified a cytoplasmic function for ATM that participates in the cellular damage response to ROS. We show that in response to elevated ROS, ATM activates the TSC2 tumor suppressor via the LKB1/AMPK metabolic pathway in the cytoplasm to repress mTORC1 and induce autophagy. Importantly, elevated ROS and dysregulation of mTORC1 in ATM-deficient cells is inhibited by rapamycin, which also rescues lymphomagenesis in Atm-deficient mice. Our results identify a cytoplasmic pathway for ROS-induced ATM activation of TSC2 to regulate mTORC1 signaling and autophagy, identifying an integration node for the cellular damage response with key pathways involved in metabolism, protein synthesis, and cell survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
mTORC1 repression by ROS in the cytoplasm (A and B) Western analysis of H2O2 treated MCF7 cells. (C) Western analysis of cytoplasmic and nuclear fractions of H2O2 treated MCF7 cells. (D) Western analysis of MCF7 cells treated with H2O2 in the presence or absence of 100 ng/mL leptomycin B. LDH and LAMIN are controls for cytoplasmic and nuclear fractions, respectively.
Fig. 2.
mTORC1 repression induces autophagy (A) MCF7 cells stably transfected with GFP-LC3 were treated with H2O2 for 1 h and analyzed by microscopy for the presence of fluorescent puncta. Cells undergoing autophagy were quantitated as a percentage of total GFP positive cells. The graph shows the total number of puncta positive cells divided by total GFP positive cells, normalized to the vehicle control [n = 2, * P < 0.001 (χ2 test)]. (B and C) Western analysis of H2O2 treated SKOV-3 cells. (D) Electron microscopy images of H2O2 treated MEFs. Arrows indicate autophagosomes.
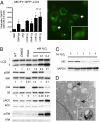
Fig. 3.
Participation of ATM in mTORC1 repression by ROS (A) Western analysis of EBV-immortalized B-lymphocytes obtained from an AT-patient (AT-B), or nonaffected individual (WT-B) were treated with H2O2 for 1 h. (B) MEFs derived from Atm+/+, Atm+/-, or _Atm_-/- mice were treated with H2O2 for 1 h. Western analyses shown above and quantitation of the response (percent change in ratio of phosphorylated S6K/total S6K setting the NT control to 100%) of independent clonal isolates from Atm+/+ (n = 3), Atm+/- (n = 3), or _Atm_-/- (n = 3) mice shown in the graph below. *P < 0.05 (t test) (C) Immunohistochemistry of thymi from _Atm_-/- mice. The thymi of _Atm_-/- mice treated with rapamycin were markedly atrophic and hypocellular, with few if any residual tumor cells apparent (Right). (D) Quantitation of Western analysis to determine the ratio of phospho-S6K/total S6K (n = 5 normal, n = 9 tumors, n = 4 tumor+rapa), and phospho-S6/total S6 (n = 7 normal, n = 12 tumors, n = 6 tumor+rapa) in thymi in response to rapamycin. *P ≤ 0.05 (Mann-Whitney test, compared to normal) (E) Kaplan-Meier survival curves for _Atm_-/- mice treated with 15 mg/kg rapamycin, and control _Atm_-/- mice and Atm+/- mice. P < 0.001 (log-rank test).
Fig. 4.
LKB1 mediates AMPK activation and mTORC1 repression by H2O2 (A) Immunoprecipitation showing that LKB1 is phosphorylated at Thr366 by ATM in response to H2O2 and 20Gy IR (positive control) in HEK293 cells. (B) Western analysis of LKB1-deficient HeLa S3 cells (parental) and stable clones expressing wild-type Lkb1 (WT) (n = 4) or T363A mutant Lkb1 (MT) (n = 2) treated with H2O2. *P < 0.03 versus parental. **P < 0.03 versus WT, 0.05 versus MT. (t test).
Fig. 5.
ROS induces AMPK activation of Tsc2 (A) Western analyses of Tsc2-proficient and deficient MEFs treated with H2O2. (B) Western analysis of HEK293 functional assay showing TSC2AMPK2A mutant is deficient in ROS-induced mTORC1 repression.
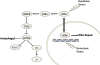
Fig. 6.
Schematic showing cytoplasmic signaling pathway from ATM to TSC2 via LKB1 and AMPK to repress mTORC1 and induce autophagy.
Similar articles
- Reactive nitrogen species regulate autophagy through ATM-AMPK-TSC2-mediated suppression of mTORC1.
Tripathi DN, Chowdhury R, Trudel LJ, Tee AR, Slack RS, Walker CL, Wogan GN. Tripathi DN, et al. Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):E2950-7. doi: 10.1073/pnas.1307736110. Epub 2013 Jul 22. Proc Natl Acad Sci U S A. 2013. PMID: 23878245 Free PMC article. - ATM engages the TSC2/mTORC1 signaling node to regulate autophagy.
Alexander A, Kim J, Walker CL. Alexander A, et al. Autophagy. 2010 Jul;6(5):672-3. doi: 10.4161/auto.6.5.12509. Epub 2010 Jul 1. Autophagy. 2010. PMID: 20581436 Free PMC article. - Differential localization of ATM is correlated with activation of distinct downstream signaling pathways.
Alexander A, Walker CL. Alexander A, et al. Cell Cycle. 2010 Sep 15;9(18):3685-6. doi: 10.4161/cc.9.18.13253. Epub 2010 Sep 5. Cell Cycle. 2010. PMID: 20890104 Free PMC article. - LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.
Shaw RJ. Shaw RJ. Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review. - The ATM protein kinase and cellular redox signaling: beyond the DNA damage response.
Ditch S, Paull TT. Ditch S, et al. Trends Biochem Sci. 2012 Jan;37(1):15-22. doi: 10.1016/j.tibs.2011.10.002. Epub 2011 Nov 11. Trends Biochem Sci. 2012. PMID: 22079189 Free PMC article. Review.
Cited by
- Annexin A1 Preferentially Predicts Poor Prognosis of Basal-Like Breast Cancer Patients by Activating mTOR-S6 Signaling.
Bhardwaj A, Ganesan N, Tachibana K, Rajapakshe K, Albarracin CT, Gunaratne PH, Coarfa C, Bedrosian I. Bhardwaj A, et al. PLoS One. 2015 May 22;10(5):e0127678. doi: 10.1371/journal.pone.0127678. eCollection 2015. PLoS One. 2015. PMID: 26000884 Free PMC article. - Differential regulatory functions of three classes of phosphatidylinositol and phosphoinositide 3-kinases in autophagy.
Yu X, Long YC, Shen HM. Yu X, et al. Autophagy. 2015;11(10):1711-28. doi: 10.1080/15548627.2015.1043076. Autophagy. 2015. PMID: 26018563 Free PMC article. Review. - Autophagy-dependent senescence in response to DNA damage and chronic apoptotic stress.
Singh K, Matsuyama S, Drazba JA, Almasan A. Singh K, et al. Autophagy. 2012 Feb 1;8(2):236-51. doi: 10.4161/auto.8.2.18600. Epub 2012 Feb 1. Autophagy. 2012. PMID: 22240589 Free PMC article. - Autophagy in acute brain injury.
Galluzzi L, Bravo-San Pedro JM, Blomgren K, Kroemer G. Galluzzi L, et al. Nat Rev Neurosci. 2016 Aug;17(8):467-84. doi: 10.1038/nrn.2016.51. Epub 2016 Jun 3. Nat Rev Neurosci. 2016. PMID: 27256553 Review. - ATM inhibition drives metabolic adaptation via induction of macropinocytosis.
Huang Z, Chen CW, Buj R, Tangudu NK, Fang RS, Leon KE, Dahl ES, Varner EL, von Krusenstiern E, Cole AR, Snyder NW, Aird KM. Huang Z, et al. J Cell Biol. 2023 Jan 2;222(1):e202007026. doi: 10.1083/jcb.202007026. Epub 2022 Nov 18. J Cell Biol. 2023. PMID: 36399181 Free PMC article.
References
- Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. - PubMed
- Elledge SJ. Cell cycle checkpoints: Preventing an identity crisis. Science. 1996;274:1664–1672. - PubMed
- Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–186. - PubMed
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. - PubMed
- Schneider JG, et al. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4:377–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK124709/DK/NIDDK NIH HHS/United States
- R01 CA21765/CA/NCI NIH HHS/United States
- CA16672/CA/NCI NIH HHS/United States
- R01 GM062694/GM/NIGMS NIH HHS/United States
- R01 CA063613/CA/NCI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- P30 ES007784/ES/NIEHS NIH HHS/United States
- R01 NS058956/NS/NINDS NIH HHS/United States
- R01 CA63613/CA/NCI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- ES007784/ES/NIEHS NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- P01 HD18655/HD/NICHD NIH HHS/United States
- R01 CA71387/CA/NCI NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- R01 CA108941/CA/NCI NIH HHS/United States
- R01 CA071387/CA/NCI NIH HHS/United States
- R01 CA143811/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous