Regulation of epithelial sodium channel trafficking by ubiquitination - PubMed (original) (raw)
Review
Regulation of epithelial sodium channel trafficking by ubiquitination
Douglas C Eaton et al. Proc Am Thorac Soc. 2010 Feb.
Abstract
Amiloride-sensitive epithelial sodium (Na(+)) channels (ENaC) play a crucial role in Na(+) transport and fluid reabsorption in the kidney, lung, and colon. The magnitude of ENaC-mediated Na(+) transport in epithelial cells depends on the average open probability of the channels and the number of channels on the apical surface of epithelial cells. The number of channels in the apical membrane, in turn, depends upon a balance between the rate of ENaC insertion and the rate of removal from the apical membrane. ENaC is made up of three homologous subunits, alpha, beta, and gamma. The C-terminal domain of all three subunits is intracellular and contains a proline rich motif (PPxY). Mutations or deletion of this PPxY motif in the beta and gamma subunits prevent the binding of one isoform of a specific ubiquitin ligase, neural precursor cell expressed developmentally down-regulated protein (Nedd4-2) to the channel in vitro and in transfected cell systems, thereby impeding ubiquitin conjugation of the channel subunits. Ubiquitin conjugation would seem to imply that ENaC turnover is determined by the ubiquitin-proteasome system, but when MDCK cells are transfected with ENaC, ubiquitin conjugation apparently leads to lysosomal degradation. However, in untransfected epithelial cells (A6) expressing endogenous ENaC, ENaC appears to be degraded by the ubiquitin-proteasome system. Nonetheless, in both transfected and untransfected cells, the rate of ENaC degradation is apparently controlled by the rate of Nedd4-2-mediated ENaC ubiquitination. Controlling the rate of degradation is apparently important enough to have multiple, redundant pathways to control Nedd4-2 and ENaC ubiquitination.
Figures
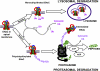
Figure 1.
A schematic diagram of epithelial sodium (Na+) channel (ENaC) degradation. Ubiquitination of proteins generally requires three distinct enzymatic activities mediated by either two or three enzymes. First, a ubiquitin-activating enzyme (E1) activates cytosolic ubiquitin by forming a high-energy thioester bond (E1-S∼ubiquitin) in an ATP-requiring reaction. Ubiquitin is then transferred to a ubiquitin-conjugating enzyme (E2), followed by the addition of ubiquitin to target proteins by a ubiquitin ligase (E3). In the case of ENaC, the E3 enzyme is neural precursor cell expressed developmentally down-regulated protein (Nedd4-2). For Hect domain E3 enzymes like Nedd4, a third high-energy thioester bond is formed between ubiquitin and a Cys residue on the E3 before its transfer to the substrate. Ubiquitin is covalently linked to ENaC via an isopeptide bond formed through its C-terminal glycine to the ɛ-amino group of lysine residues. Additional ubiquitins can be added to the first using the terminal carboxylic group of glycine 76 on one ubiquitin and lysine 48 of another ubiquitin molecule, resulting in a chain of at least four or five ubiquitins, a process known as polyubiquitination (lower pathway). In contrast, single ubiquitin molecules can be added to different lysine residues on the target protein so that the final stoichiometry is one ubiquitin per lysine on multiple lysines on the target protein, a process known as monoubiquitination (upper pathway). Monoubiquitinated ENaC is trafficked to and degraded in the lysosomes, whereas polyubiquitinated ENaC is recognized and degraded by the proteasome. The data indicate that in cells expressing endogenous ENaC subunits, polyubiquitination is predominant and ubiquitin-conjugated channels are degraded by proteasomes; in contrast, in cells overexpressing ENaC subunits, monoubiquitination is likely and ubiquitin-conjugated ENaC molecules are often degraded in lysosomes.
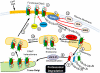
Figure 2.
A schematic of ENaC trafficking and regulation by Nedd4-2–mediated ubiquitination. αβγ ENaC heterotrimers are assembled, exit the ER through the Golgi. They then traffic through a post-Golgi compartment (step 1) before entering the apical membrane in phosphatidyl-inositol-rich lipid rafts (step 2). These channels are functional and responsible for most, if not all, Na+ transport (step 3). The functional heterotrimers in lipid rafts are associated with and ubiquitinated by an ENaC-specific ubiquitin ligase, Nedd4. Ubiquitination promotes internalization of the αβγ trimers into a subapical, sorting compartment (step 5). ENaC can suffer two fates within this compartment. One fate is to be de-ubiquitinated by the enzyme, UCH-L3, and returned to a post-Golgi recycling compartment (step 9) from which they can return to the surface membrane (step 10). The other fate is to be disassembled into individual, ubiquitinated subunits (step 6). Some of the ubiquitinated subunits will be recognized by the 26S proteasome and degraded (step 7), while others will be de-ubiquitinated by ubiquitin-specific proteases (like USP2-45) and trafficked back to the Golgi (step 8). Once disassembled, the fate of the subunits is apparently different: β and γ are more likely to be degraded by the proteasome (a few hours after internalization) while α is more likely to be trafficked back to the Golgi and, therefore, remain undegraded for long periods (tens of hours). Besides the removal of ubiquitin by de-ubiquitinating enzymes, Nedd4-2–mediated ubiquitination can be regulated in several ways by. Phosphorylation of ENaC plays a role in altering Nedd4 ubiquitination (step 11). Mitogen-activated protein kinase (MAPK) phosphorylation of ENaC promotes Nedd4-2 binding and enhanced retrieval and degradation. In contrast, casein kinase 2 (CK II) phosphorylates ENaC and prevents interaction with Nedd4, thereby reducing ubiquitination, retrieval, and degradation. Phosphorylation of Nedd4-2 itself plays a role in altering ENaC ubiquitination (step 12). Serum and glucocorticoid-dependent kinase (SGK) can phosphorylate Nedd4-2, which when phosphorylated has a lower affinity for binding to the PPxY motif in ENaC. This effect is apparently caused by the phosphorylated form of Nedd4-2 interacting strongly with the scaffold protein, 14-3-3β and ɛ, that prevents Nedd4-2 interaction with ENaC. G protein receptor kinase 2 (GRK2) also phosphorylates Nedd4-2 to reduce interaction with ENaC and prevent ubiquitination and degradation and, finally, Nedd4-2 is phosphorylated and inhibited by cAMP-dependent protein kinase A.
Similar articles
- Regulation of epithelial sodium channels by the ubiquitin-proteasome proteolytic pathway.
Malik B, Price SR, Mitch WE, Yue Q, Eaton DC. Malik B, et al. Am J Physiol Renal Physiol. 2006 Jun;290(6):F1285-94. doi: 10.1152/ajprenal.00432.2005. Am J Physiol Renal Physiol. 2006. PMID: 16682484 Review. - Role of Nedd4-2 and polyubiquitination in epithelial sodium channel degradation in untransfected renal A6 cells expressing endogenous ENaC subunits.
Malik B, Yue Q, Yue G, Chen XJ, Price SR, Mitch WE, Eaton DC. Malik B, et al. Am J Physiol Renal Physiol. 2005 Jul;289(1):F107-16. doi: 10.1152/ajprenal.00179.2002. Epub 2005 Mar 15. Am J Physiol Renal Physiol. 2005. PMID: 15769939 - Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination.
Staub O, Abriel H, Plant P, Ishikawa T, Kanelis V, Saleki R, Horisberger JD, Schild L, Rotin D. Staub O, et al. Kidney Int. 2000 Mar;57(3):809-15. doi: 10.1046/j.1523-1755.2000.00919.x. Kidney Int. 2000. PMID: 10720933 Review. - Hypoxia-induced inhibition of epithelial Na(+) channels in the lung. Role of Nedd4-2 and the ubiquitin-proteasome pathway.
Gille T, Randrianarison-Pellan N, Goolaerts A, Dard N, Uzunhan Y, Ferrary E, Hummler E, Clerici C, Planès C. Gille T, et al. Am J Respir Cell Mol Biol. 2014 Mar;50(3):526-37. doi: 10.1165/rcmb.2012-0518OC. Am J Respir Cell Mol Biol. 2014. PMID: 24093724 - Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination.
Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Staub O, et al. EMBO J. 1997 Nov 3;16(21):6325-36. doi: 10.1093/emboj/16.21.6325. EMBO J. 1997. PMID: 9351815 Free PMC article.
Cited by
- δ ENaC: a novel divergent amiloride-inhibitable sodium channel.
Ji HL, Zhao RZ, Chen ZX, Shetty S, Idell S, Matalon S. Ji HL, et al. Am J Physiol Lung Cell Mol Physiol. 2012 Dec 15;303(12):L1013-26. doi: 10.1152/ajplung.00206.2012. Epub 2012 Sep 14. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22983350 Free PMC article. Review. - USP18 Sensitivity of Peptide Transporters PEPT1 and PEPT2.
Warsi J, Hosseinzadeh Z, Elvira B, Pelzl L, Shumilina E, Zhang DE, Lang KS, Lang PA, Lang F. Warsi J, et al. PLoS One. 2015 Jun 5;10(6):e0129365. doi: 10.1371/journal.pone.0129365. eCollection 2015. PLoS One. 2015. PMID: 26046984 Free PMC article. - The Role of Intercalated Cell Nedd4-2 in BP Regulation, Ion Transport, and Transporter Expression.
Nanami M, Pham TD, Kim YH, Yang B, Sutliff RL, Staub O, Klein JD, Lopez-Cayuqueo KI, Chambrey R, Park AY, Wang X, Pech V, Verlander JW, Wall SM. Nanami M, et al. J Am Soc Nephrol. 2018 Jun;29(6):1706-1719. doi: 10.1681/ASN.2017080826. Epub 2018 May 17. J Am Soc Nephrol. 2018. PMID: 29773687 Free PMC article. - Identification of Cysteine Ubiquitylation Sites on the Sec23A Protein of the COPII Complex Required for Vesicle Formation from the ER.
Amodio G, Margarucci L, Moltedo O, Casapullo A, Remondelli P. Amodio G, et al. Open Biochem J. 2017 Apr 28;11:36-46. doi: 10.2174/1874091X01711010036. eCollection 2017. Open Biochem J. 2017. PMID: 28553408 Free PMC article. - Mechanistic target of rapamycin (mTOR) regulates trophoblast folate uptake by modulating the cell surface expression of FR-α and the RFC.
Rosario FJ, Powell TL, Jansson T. Rosario FJ, et al. Sci Rep. 2016 Aug 26;6:31705. doi: 10.1038/srep31705. Sci Rep. 2016. PMID: 27562465 Free PMC article.
References
- Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU. Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life 2008;60:620–628. - PubMed
- Babini E, Geisler HS, Siba M, Grunder S. A new subunit of the epithelial Na+ channel identifies regions involved in Na+ self-inhibition. J Biol Chem 2003;278:28418–28426. - PubMed
- Ji HL, Su XF, Kedar S, Li J, Barbry P, Smith PR, Matalon S, Benos DJ. Delta -subunit confers novel biophysical features to alpha beta gamma -human ENaC via a physical interaction. J Biol Chem 2006;281:8233–8241. - PubMed
- Ji HL, Bishop LR, Anderson SJ, Fuller CM, Benos DJ. The role of pre-H2 domains of alpha- and delta-epithelial Na+ channels in ion permeation, conductance, and amiloride sensitivity. J Biol Chem 2004;279:8428–8440. - PubMed
- Biasio W, Chang T, McIntosh CJ, McDonald FJ. Identification of Murr1 as a regulator of the human delta epithelial sodium channel. J Biol Chem 2004;279:5429–5434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources