The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury - PubMed (original) (raw)
The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury
Yoichiro Uchida et al. Transplantation. 2010.
Abstract
BACKGROUND.: A neutrophil elastase (NE) inhibitor, Sivelestat, has been approved for the treatment of acute lung injury associated with systemic inflammation in humans. Some reports have also shown its protective effects in liver inflammatory states. We have recently documented the importance of NE in the pathophysiology of liver ischemia/reperfusion injury, a local Ag-independent inflammation response. This study was designed to explore putative cytoprotective functions of clinically available Sivelestat in liver ischemia/reperfusion injury. METHODS.: Partial warm ischemia was produced in the left and middle hepatic lobes of C57BL/6 mice for 90 min, followed by 6 or 24 hr of reperfusion. The mice were given Sivelestat (100 mg/kg, subcutaneous) at 10 min before ischemia, 10 min before reperfusion, and at 1 and 3 hr of reperfusion thereafter. RESULTS.: Sivelestat treatment significantly reduced serum alanine aminotransferase levels and NE activity, when compared with controls. Histological liver examination has revealed that unlike in controls, Sivelestat ameliorated the hepatocellular damage and decreased local neutrophil activity and infiltration. The expression of proinflammatory cytokines (tumor necrosis factor-alpha and interleukin-6), chemokines (CXCL-1, CXCL-2, and CXCL-10), and toll-like receptor 4 was significantly reduced in the treatment group, along with diminished apoptosis through caspase-3 pathway. Moreover, in vitro studies confirmed downregulation of proinflammatory cytokine and chemokine programs in mouse macrophage cell cultures, along with depression of innate toll-like receptor 4 signaling. CONCLUSION.: Sivelestat-mediated NE inhibition may represent an effective therapeutic option in liver transplantation and other inflammation disease states.
Figures
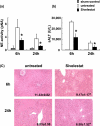
Figure 1
The hepatocellular damage. (a) Sivelestat treatment inhibited NE activity in mouse serum after liver warm ischemia (90 min) followed by reperfusion (6 and 24 h) (*P<0.01; n=4-6/group). (b) Serum ALT levels at both 6 and 24 h after reperfusion were lower in Sivelestat treatment group, as compared with untreated controls (*P<0.01; n=6/group). Means and SD are shown. (c) Representative liver histology (HE staining; X100 magnification) of ischemic liver lobes harvested at 6 and 24 h after reperfusion (Upper panel: 6 h; Suzuki's score = 11.33 ± 0.82 vs 9.17 ± 1.17; p<0.01, Lower panel: 24 h; 8.83 ± 0.98 vs 6.50 ± 1.52; *P<0.01, n=6/group).
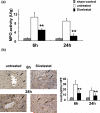
Figure 2
Neutrophil accumulation. (a) MPO, an index of neutrophil infiltration, activity was markedly reduced in the treatment group (**P<0.05 ; n=3/group). (b) Sivelestat-treated livers showed significantly decreased numbers of infiltrated polymorphonuclear cells stained by Ly-6G (dark brown spots) as compared with untreated controls (X400 magnification **P < 0.05; n=3/group). Means and SD are shown.
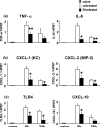
Figure 3
RT-PCR-assisted detection of pro-inflammatory mediators in IRI livers. (a) Cytokine gene expression. (b) Chemokine gene expression. (c) TLR4 and CXCL-10 gene expression in untreated and treated livers. Data were normalized to HPRT gene expression (*p < 0.01, **p < 0.05; n =3/group). Means and SD are shown.
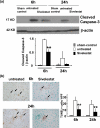
Figure 4
Apoptosis in ischemic livers. (a) Treatment with Sivelestat significantly reduced cleaved Caspase-3 expression by Western blots (**P<0.05; n=2-3/group). (b) Sivelestat-treated livers showed significantly decreased numbers of TUNEL positive cells (dark brown spots) as compared with untreated controls (X400 magnification, **P<0.05; n=3/group). Means and SD are shown.
Figure 5
RT-PCR-assisted detection of pro-inflammatory mediators in mouse macrophage (RAW 264.7) cell cultures. Cells were pretreated with Sivelestat (100 μM) 10 and 30 min prior to LPS (10 ng/ml), and 1 and 2.5 h after the stimulation. Cells were harvested at 4 h. (a) Cytokine gene expression. (b) Chemokine gene expression. (c) TLR4 and CXCL-10 gene expression, as compared with LPS alone. Data were normalized to HPRT gene expression (*p < 0.01, **p < 0.05). Means and SD are shown.
Figure 6
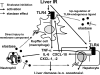
Cross-talk between NE and inflammation responses in liver IRI. Activated Kupffer cells produce pro-inflammatory cytokines (TNF-α and IL-6) and chemokines (CXCL-1 and CXCL-2). Moreover, activation of TLR4 on Kupffer cells and hepatocytes accelerates the production of its downstream CXCL-10. TNF-α affects surrounding hepatocytes causing their apoptosis, and triggers neutrophil-attracting CXC chemokines. The activated neutrophil-derived NE induces inflammatory chemokine (CXCL-1, CXCL-2) expression by neutrophils and accelerates IR-mediated damage due to the feedback with recruited neutrophils, resulting in the direct injury to membrane components. NE may also serve as a putative endogenous TLR4 ligand, causing TLR4 up-regulation on Kupffer cells/hepatocytes. Sivelestat may also inhibit the inflammatory mediators through NF-kB inhibition on Kupffer cells and hepatocytes.
Comment in
- Effects of neutrophil elastase inhibitor on progression of acute lung injury after liver transplantation.
Kaido T, Uemoto S. Kaido T, et al. Transplantation. 2010 Aug 15;90(3):335-7. doi: 10.1097/TP.0b013e3181e49bf2. Transplantation. 2010. PMID: 20683430 No abstract available.
References
- Farmer DG, Amersi F, Kupiec-Weglinski JW, Busuttil RW. Current status of ischemia and reperfusion injury in the liver. Transplant Rev. 2000;14:106.
- Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: Pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891. - PubMed
- O'Neill LA. Therapeutic targeting of Toll-like receptors for inflammatory and infectious diseases. Curr Opin Pharmacol. 2003;3:396. - PubMed
- Obhrai J, Goldstein DR. The role of toll-like receptors in solid organ transplantation. Transplantation. 2006;81:497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI023847/AI/NIAID NIH HHS/United States
- AI23847/AI/NIAID NIH HHS/United States
- R01 AI042223/AI/NIAID NIH HHS/United States
- R56 AI023847/AI/NIAID NIH HHS/United States
- R01 DK062357/DK/NIDDK NIH HHS/United States
- R21 AI023847/AI/NIAID NIH HHS/United States
- AI42223/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials