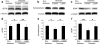Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes - PubMed (original) (raw)
Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes
M Sasaki et al. Diabetologia. 2010 May.
Abstract
Aims/hypothesis: Diabetic retinopathy is a progressive neurodegenerative disease, but the underlying mechanism is still obscure. Here, we focused on oxidative stress in the retina, and analysed its influence on retinal neurodegeneration, using an antioxidant, lutein.
Methods: C57BL/6 mice with streptozotocin-induced diabetes were constantly fed either a lutein-supplemented diet or a control diet from the onset of diabetes, and their metabolic data were recorded. In 1-month-diabetic mice, reactive oxygen species (ROS) in the retina were measured using dihydroethidium and visual function was evaluated by electroretinograms. Levels of activated extracellular signal-regulated kinase (ERK), synaptophysin and brain-derived neurotrophic factor (BDNF) were also measured by immunoblotting in the retina of 1-month-diabetic mice. In the retinal sections of 4-month-diabetic mice, histological changes, cleaved caspase-3 and TUNEL staining were analysed.
Results: Lutein did not affect the metabolic status of the diabetic mice, but it prevented ROS generation in the retina and the visual impairment induced by diabetes. ERK activation, the subsequent synaptophysin reduction, and the BDNF depletion in the diabetic retina were all prevented by lutein. Later, in 4-month-diabetic mice, a decrease in the thickness of the inner plexiform and nuclear layers, and ganglion cell number, together with increase in cleaved caspase-3- and TUNEL-positive cells, were avoided in the retina of lutein-fed mice.
Conclusions/interpretation: The results indicated that local oxidative stress that has a neurodegenerative influence in the diabetic retina is prevented by constant intake of a lutein-supplemented diet. The antioxidant, lutein may be a potential therapeutic approach to protect visual function in diabetes.
Figures
Fig. 1
Diabetes-induced oxidative stress in the retina was reduced by lutein. a DHE staining in the retinal section. Original magnification ×200; scale bar, 30 μm. b Fluorescence intensity in the INL relative to that of non-diabetic mice, measured by the Image J program. Note that ROS increased throughout the retina of mice diabetic for 1 month; however, this change was prevented by constant intake of lutein from the onset of diabetes. Non-diabetic mice, _n_=4; diabetic mice fed control diet, _n_=4; diabetic mice fed lutein diet, _n_=5. Values are means±SD. *p < 0.05, **p < 0.01
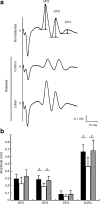
Fig. 2
Diabetes-induced visual dysfunction was suppressed by lutein. Representative wave responses from an individual mouse in each group to one flash (a). Constant lutein intake from the onset of diabetes significantly inhibited the reduction of OP3 and total OPs (ΣOPs; summation of OP2, 3, and 4) amplitude in 1-month-diabetic mice (b). Black columns, non-diabetic mice, _n_=5; white columns, diabetic mice fed control diet, _n_=6; grey columns, diabetic mice fed lutein diet, _n_=6. Values are means±SD. *p < 0.05
Fig. 3
Diabetes-induced biochemical changes in the retina are prevented by lutein as shown by immunoblot analysis. a, d Diabetes-induced ERK activation detected by ERK phosphorylation (pERK) in the retina of 1-month-diabetic mice was prevented by constant intake of lutein. Non-diabetic mice, _n_=8; diabetic mice fed control diet, _n_=8; diabetic mice fed lutein diet, _n_=7. b, e A decrease in synaptophysin in the retina of 1-month-diabetic mice was prevented by constant intake of lutein. Non-diabetic mice, _n_=6; diabetic mice fed control diet, _n_=6; diabetic mice fed lutein diet, _n_=6. c, f The level of BDNF was also reduced in the retina of 1-month-diabetic mice, and was significantly rescued by constant intake of lutein. Non-diabetic, mice _n_=6; diabetic mice fed control diet, _n_=6; diabetic mice fed lutein diet, _n_=6. Bar graph values are relative to those of the non-diabetic controls. Values are means±SD. *p < 0.05, **p < 0.01
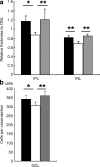
Fig. 4
Diabetes-induced histological changes were suppressed by lutein. Thickness of each retinal layer was measured in paraffin sections after haematoxylin and eosin staining. Original magnification ×400. a Relative thicknesses of IPL and INL, normalised to ONL measured at the same point, respectively, were reduced in the retina of 4-month-diabetic mice, but remained normal in the diabetic mice fed the lutein diet all the time from diabetes onset. b The neuronal cell number in the GCL in one cross-section of the mice diabetic for 4 months was decreased; however, this change was significantly suppressed by lutein. Black columns, non-diabetic mice, _n_=6; white columns, diabetic mice fed control diet, _n_=5; grey columns, diabetic mice fed lutein diet, _n_=5. Values are means±SD. *p < 0.05, **p < 0.01
Fig. 5
Diabetes-induced apoptosis was inhibited by lutein. a Caspase-3 activation detected by immunohistochemistry of cleaved caspase-3. Caspase-3 activation was obvious in GCL and weak in INL cells of 4-month-diabetic retinas (arrowheads, cleaved caspase-3-positive cells); however, the activation was suppressed by constant intake of lutein. b TUNEL staining in the GCL of 4-month-diabetic retinas (arrowheads) was also suppressed by lutein treatment. Cleaved caspase-3-positive cells (c) and the TUNEL-positive cells (d) in GCL were counted. a, c Non-diabetic mice, _n_=4; diabetic mice fed control diet, _n_=4; diabetic mice fed lutein diet, _n_=4. b, d Non-diabetic mice, _n_=5; diabetic mice fed control diet, _n_=6; diabetic mice fed lutein diet, _n_=6. Value are means±SD. *p < 0.05, **p < 0.01. a Original magnification ×200, scale bar, 30 μm. b Original magnification ×400; scale bar, 40 μm
Similar articles
- Beneficial effect of docosahexanoic acid and lutein on retinal structural, metabolic, and functional abnormalities in diabetic rats.
Arnal E, Miranda M, Johnsen-Soriano S, Alvarez-Nölting R, Díaz-Llopis M, Araiz J, Cervera E, Bosch-Morell F, Romero FJ. Arnal E, et al. Curr Eye Res. 2009 Nov;34(11):928-38. doi: 10.3109/02713680903205238. Curr Eye Res. 2009. PMID: 19958109 - Hydrogen-rich saline prevents early neurovascular dysfunction resulting from inhibition of oxidative stress in STZ-diabetic rats.
Feng Y, Wang R, Xu J, Sun J, Xu T, Gu Q, Wu X. Feng Y, et al. Curr Eye Res. 2013 Mar;38(3):396-404. doi: 10.3109/02713683.2012.748919. Epub 2012 Dec 19. Curr Eye Res. 2013. PMID: 23252792 - Environmental enrichment protects the retina from early diabetic damage in adult rats.
Dorfman D, Aranda ML, González Fleitas MF, Chianelli MS, Fernandez DC, Sande PH, Rosenstein RE. Dorfman D, et al. PLoS One. 2014 Jul 8;9(7):e101829. doi: 10.1371/journal.pone.0101829. eCollection 2014. PLoS One. 2014. PMID: 25004165 Free PMC article. - Neural degeneration in the retina of the streptozotocin-induced type 1 diabetes model.
Ozawa Y, Kurihara T, Sasaki M, Ban N, Yuki K, Kubota S, Tsubota K. Ozawa Y, et al. Exp Diabetes Res. 2011;2011:108328. doi: 10.1155/2011/108328. Epub 2011 Nov 17. Exp Diabetes Res. 2011. PMID: 22144984 Free PMC article. Review. - Neuroprotective effects of lutein in the retina.
Ozawa Y, Sasaki M, Takahashi N, Kamoshita M, Miyake S, Tsubota K. Ozawa Y, et al. Curr Pharm Des. 2012;18(1):51-6. doi: 10.2174/138161212798919101. Curr Pharm Des. 2012. PMID: 22211688 Free PMC article. Review.
Cited by
- Neuroprotective effects of lutein in a rat model of retinal detachment.
Woo TT, Li SY, Lai WW, Wong D, Lo AC. Woo TT, et al. Graefes Arch Clin Exp Ophthalmol. 2013 Jan;251(1):41-51. doi: 10.1007/s00417-012-2128-z. Epub 2012 Aug 18. Graefes Arch Clin Exp Ophthalmol. 2013. PMID: 22899456 Free PMC article. - Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats.
Si YF, Wang J, Guan J, Zhou L, Sheng Y, Zhao J. Si YF, et al. Br J Pharmacol. 2013 Jun;169(3):619-31. doi: 10.1111/bph.12163. Br J Pharmacol. 2013. PMID: 23488985 Free PMC article. - Expression of neuroglobin in ocular hypertension induced acute hypoxic-ischemic retinal injury in rats.
Shi SY, Feng XM, Li Y, Li X, Chen XL. Shi SY, et al. Int J Ophthalmol. 2011;4(4):393-5. doi: 10.3980/j.issn.2222-3959.2011.04.14. Epub 2011 Aug 18. Int J Ophthalmol. 2011. PMID: 22553688 Free PMC article. - Diabetic choriocapillaris flow deficits affect the outer retina and are related to hemoglobin A1c and systolic blood pressure levels.
Nagai N, Mushiga Y, Ozawa Y. Nagai N, et al. Sci Rep. 2023 Dec 19;13(1):22570. doi: 10.1038/s41598-023-50132-1. Sci Rep. 2023. PMID: 38114663 Free PMC article. - MST1-knockdown protects against impairment of working memory via regulating neural activity in depression-like mice.
Chen B, Zhang Q, Yan Y, Zhang T. Chen B, et al. Genes Brain Behav. 2022 Feb;21(2):e12782. doi: 10.1111/gbb.12782. Epub 2022 Jan 19. Genes Brain Behav. 2022. PMID: 35044088 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous