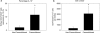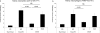Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney - PubMed (original) (raw)
Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney
Gilbert R Kinsey et al. Kidney Int. 2010 May.
Abstract
Reperfusion following ischemia is associated with acute kidney injury and inflammation. Using a mouse model, we exposed the kidney to a nonlethal period of ischemia, rendering it refractory to future ischemia-induced dysfunction. This ischemic preconditioning is partially mediated by Treg lymphocytes that suppress immune responses. We found that this maneuver significantly inhibited the accumulation of neutrophils and macrophages, tubular necrosis, and loss of kidney function caused by a subsequent ischemia/reperfusion injury 1 week later. The initial ischemia/reperfusion caused a significant increase in CD4(+)CD25(+)FoxP3(+) and CD4(+)CD25(+)IL-10(+) Treg cells within the kidney at 7 days of reperfusion. Treatment of preconditioned mice with a Treg cell-depleting antibody (PC61) reversed the effect of preconditioning on kidney neutrophil accumulation and partially inhibited the functional and histological protection of preconditioning. Adoptive transfer of Treg cells in naive mice, before ischemia/reperfusion, mimicked the protective and anti-inflammatory effects of ischemic preconditioning on the kidney. These studies highlight the role of Treg cells in ischemic preconditioning.
Figures
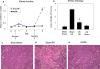
Figure 1. Ischemic preconditioning prevents ischemia-reperfusion-induced kidney dysfunction and acute tubular necrosis
C57Bl/6 mice underwent sham surgery or bilateral renal pedicle clamping (IRI) for 24 min on day 0 and were allowed to recover for 7 days. On day 7 mice underwent 28 min IRI. Plasma creatinine was measured periodically to assess renal function (A). Twenty four hrs after the second surgery, kidney sections were fixed and stained with H&E to assess outer medulla acute tubular necrosis (ATN) in the different treatment groups (B), pictures are representative of at least 8 mice per group (C,D,E). Data are presented as the mean ± SEM; * - P<0.01 compared to Sham/Sham control group; # - P<0.01 compared to Sham/IRI, non-preconditioned group.
Figure 2. Ischemic preconditioning inhibits innate leukocyte accumulation after IRI
Twenty four hours after the 2nd surgery, kidneys were harvested, digested and stained with neutrophil or macrophage markers and analyzed by flow cytometry as described in the methods section. The absolute number of neutrophils (A) and macrophages (B) per gram kidney in each group is represented graphically. Frozen kidney sections were stained with an antibody that recognizes neutrophils and recently emigrated monocytes (7/4) to assess renal accumulation in the different treatment groups, pictures are representative of at least 3 mice per group. Data are presented as the mean + SEM; * - P< 0.01 vs. Sham/Sham, control mice; # - P<0.05 compared to Sham/IRI, non-preconditioned group.
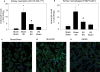
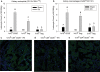
Figure 3. Kidney ischemia reperfusion injury causes kidney regulatory T cell accumulation
At 3, 7 and 14 days after 24 min IRI (preconditioned) or sham (non-preconditioned) surgery, mouse kidneys and spleen were harvested, digested and stained for Treg cell markers as described in the Methods section. The absolute number of Treg cells per gram in preconditioned mice is represented as a percentage of their respective sham control (A). Data are presented as the mean + SEM, * - P< 0.05, compared to non-preconditioned mice, n = 4–8 per group. Frozen sections from sham-operated (B) or 24 min IRI (C,D) kidneys were stained with anti-mouse CD4 (red) and anti-mouse FoxP3 (green) antibodies. Images are from the outer medulla and representative of at least 3 separate animals in each group.
Figure 4. Kidney ischemia reperfusion injury causes increased IL-10 production by Treg cells
At 7 days after 24 min IRI (preconditioned) or sham (non-preconditioned) surgery, mouse kidneys harvested, digested and stained for Treg cell surface markers CD4 and CD25, and stained intracellularly for the cytokine IL-10 as described in the Methods section. The percentage (A) and absolute number (B) of IL-10+ Treg cells per kidney in preconditioned and non-preconditioned mice is represented as the mean + SEM, * - P< 0.05, compared to non-preconditioned mice, n = 6 per group.
Figure 5. PC61 administration significantly reduces kidney Treg cells in preconditioned mice
Preconditioned mice were injected with the α-CD25 monoclonal antibody, PC61, or control IgG on day 2 of the IPC protocol (A). On day 7 of reperfusion, kidney Treg cell numbers were determined by flow cytometry (B). Data are presented as the mean + SEM; * - P< 0.01 vs. IRI/IRI-IgG.
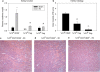
Figure 6. Treg cell depletion is associated with inhibition of the anti-inflammatory effects of ischemic preconditioning
Preconditioned mice were injected with the α-CD25 monoclonal antibody, PC61, or control IgG on day 2 of the IPC protocol. On day 8 (24 hr after the second surgery), kidney neutrophil (CD45+7-AAD−CD11b+GR-1high; A) and macrophage (CD45+7-AAD−F4/80intCD11b+; B) accumulation was assessed by flow cytometry. Data are presented as the mean + SEM; n.s. – not significant.
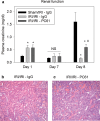
Figure 7. Treg cell depletion inhibits the kidney functional and histological protection of ischemic preconditioning
Renal function was assessed by measuring plasma creatinine at 24 hr after the first surgery (Day 1), at the time of the second surgery (Day 7) and 24 hr after the second surgery (Day 8) in non-preconditioned mice treated with IgG or preconditioned mice treated with IgG or PC61 (A). Twenty four hrs after the second surgery, kidney sections were fixed and stained with H&E to assess outer medulla tubular necrosis in preconditioned mice treated with IgG (B) or PC61 (C), pictures are representative of at least 4 mice per group. Data are presented as the mean + SEM; * - P<0.05 compared to Sham/IRI-IgG; # - P<0.01 compared to IRI/IRI-IgG; n.s. – not significant.
Figure 8. Adoptive transfer of regulatory T cells protects the kidney from subsequent ischemia-reperfusion injury
Eighteen hr prior to 28 min bilateral renal ischemia, naïve C57Bl/6 mice were administered either 1 × 106 CD4+CD25− non-Treg lymphocytes (CD25−), 1 × 105 or 1 × 106 CD4+CD25+ Treg cells by tail vein injection. Twenty four hr after sham surgery or IRI, renal function was assessed by measuring plasma creatinine levels (A). Twenty four hr after IRI kidney sections were fixed and stained with H&E to assess outer medulla tubular necrosis in the different treatment groups (B,C,D,E). Data are presented as the mean + SEM; * - P<0.05 compared to Sham; # - P<0.01 compared to CD25− - IRI; ** - P<0.001 compared to CD25− - IRI; n.d. – not done.
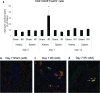
Figure 9. Adoptive transfer of regulatory T cells prevents ischemia-reperfusion-induced inflammation
Eighteen hr prior to 28 min bilateral renal ischemia, naïve C57Bl/6 mice were administered either 1 × 106 CD4+CD25− non-Treg lymphocytes (CD25−), 1 × 105 or 1 × 106 CD4+CD25+ Treg cells by tail vein injection. Twenty four hr after sham surgery or IRI, kidney neutrophil (A) and macrophage (B) accumulation was measured by flow cytometry. Frozen kidney sections were stained with an antibody that recognizes neutrophils and recently emigrated monocytes (7/4) to assess renal accumulation in the different treatment groups (C,D,E). Data are presented as the mean + SEM, means with different superscripts are significantly different from each other, P<0.01; n.d. – not done.
Similar articles
- Delayed Ischemic Preconditioning Attenuated Renal Ischemia-Reperfusion Injury by Inhibiting Dendritic Cell Maturation.
Zhang T, Song N, Fang Y, Teng J, Xu X, Hu J, Zhang P, Chen R, Lu Z, Yu X, Ding X. Zhang T, et al. Cell Physiol Biochem. 2018;46(5):1807-1820. doi: 10.1159/000489366. Epub 2018 Apr 25. Cell Physiol Biochem. 2018. PMID: 29705777 - Delayed ischaemic preconditioning in the presence of galectin-9 protects against renal ischaemic injury through a regulatory T-cell dependent mechanism.
Zhang BY, Fang Y, Jiao XY, Wu S, Cai JR, Zou JZ, Ding XQ. Zhang BY, et al. Nephrology (Carlton). 2016 Oct;21(10):828-34. doi: 10.1111/nep.12680. Nephrology (Carlton). 2016. PMID: 26609639 - The heat-shock protein-70-induced renoprotective effect is partially mediated by CD4+ CD25+ Foxp3 + regulatory T cells in ischemia/reperfusion-induced acute kidney injury.
Kim MG, Jung Cho E, Won Lee J, Sook Ko Y, Young Lee H, Jo SK, Cho WY, Kim HK. Kim MG, et al. Kidney Int. 2014 Jan;85(1):62-71. doi: 10.1038/ki.2013.277. Epub 2013 Jul 24. Kidney Int. 2014. PMID: 23884338 - Remote ischemic preconditioning for kidney protection: GSK3β-centric insights into the mechanism of action.
Liu Z, Gong R. Liu Z, et al. Am J Kidney Dis. 2015 Nov;66(5):846-56. doi: 10.1053/j.ajkd.2015.06.026. Epub 2015 Aug 10. Am J Kidney Dis. 2015. PMID: 26271146 Free PMC article. Review. - Opioids Preconditioning Upon Renal Function and Ischemia-Reperfusion Injury: A Narrative Review.
Palomino J, Echavarria R, Franco-Acevedo A, Moreno-Carranza B, Melo Z. Palomino J, et al. Medicina (Kaunas). 2019 Aug 23;55(9):522. doi: 10.3390/medicina55090522. Medicina (Kaunas). 2019. PMID: 31443610 Free PMC article. Review.
Cited by
- CD8 + CD103 + iTregs protect against ischemia-reperfusion-induced acute kidney Injury by inhibiting pyroptosis.
Chen Q, Zhang X, Yang H, Luo G, Zhou X, Xu Z, Xu A. Chen Q, et al. Apoptosis. 2024 Oct;29(9-10):1709-1722. doi: 10.1007/s10495-024-02001-z. Epub 2024 Jul 28. Apoptosis. 2024. PMID: 39068624 - VX-702 Ameliorates the Severity of Sepsis-Associated Acute Kidney Injury by Downregulating Inflammatory Factors in Macrophages.
Han Y, Wang J, Zhang J, Zheng X, Jiang Y, Liu W, Li W. Han Y, et al. J Inflamm Res. 2024 Jun 21;17:4037-4054. doi: 10.2147/JIR.S464018. eCollection 2024. J Inflamm Res. 2024. PMID: 38919509 Free PMC article. - Double-negative T cells have a reparative role after experimental severe ischemic acute kidney injury.
Lee K, Gharaie S, Kurzhagen JT, Newman-Rivera AM, Arend LJ, Noel S, Rabb H. Lee K, et al. Am J Physiol Renal Physiol. 2024 Jun 1;326(6):F942-F956. doi: 10.1152/ajprenal.00376.2023. Epub 2024 Apr 18. Am J Physiol Renal Physiol. 2024. PMID: 38634135 - The role of non-protein-coding RNAs in ischemic acute kidney injury.
Sabet Sarvestani F, Afshari A, Azarpira N. Sabet Sarvestani F, et al. Front Immunol. 2024 Feb 8;15:1230742. doi: 10.3389/fimmu.2024.1230742. eCollection 2024. Front Immunol. 2024. PMID: 38390339 Free PMC article. Review. - Mesenchymal stromal cells secretome restores bioenergetic and redox homeostasis in human proximal tubule cells after ischemic injury.
Faria J, Calcat-I-Cervera S, Skovronova R, Broeksma BC, Berends AJ, Zaal EA, Bussolati B, O'Brien T, Mihăilă SM, Masereeuw R. Faria J, et al. Stem Cell Res Ther. 2023 Dec 10;14(1):353. doi: 10.1186/s13287-023-03563-6. Stem Cell Res Ther. 2023. PMID: 38072933 Free PMC article.
References
- Burne-Taney MJ, Ascon DB, Daniels F, et al. B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol. 2003;171:3210–3215. - PubMed
- Day YJ, Huang L, Ye H, et al. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol. 2006;176:3108–3114. - PubMed
- Day YJ, Huang L, Ye H, et al. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol. 2005;288:F722–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32DK072922-01/DK/NIDDK NIH HHS/United States
- T32 DK072922-01/DK/NIDDK NIH HHS/United States
- P01 HL073361-01A19001/HL/NHLBI NIH HHS/United States
- R01 DK085259/DK/NIDDK NIH HHS/United States
- DK56223/DK/NIDDK NIH HHS/United States
- R01 DK056223-05/DK/NIDDK NIH HHS/United States
- R01 DK062324/DK/NIDDK NIH HHS/United States
- P01HL07336/HL/NHLBI NIH HHS/United States
- R01 DK056223/DK/NIDDK NIH HHS/United States
- R44 DK058413/DK/NIDDK NIH HHS/United States
- P01 HL073361/HL/NHLBI NIH HHS/United States
- R41 DK058413/DK/NIDDK NIH HHS/United States
- R41 DK058413-01/DK/NIDDK NIH HHS/United States
- DK62324/DK/NIDDK NIH HHS/United States
- F32 DK083185/DK/NIDDK NIH HHS/United States
- F32 DK083185-01/DK/NIDDK NIH HHS/United States
- F32DK083185/DK/NIDDK NIH HHS/United States
- R01 DK062324-05/DK/NIDDK NIH HHS/United States
- T32 DK072922/DK/NIDDK NIH HHS/United States
- DK58413/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials