The neurovascular unit in the setting of stroke - PubMed (original) (raw)
Review
The neurovascular unit in the setting of stroke
G J del Zoppo. J Intern Med. 2010 Feb.
Abstract
Microvessels and neurons respond rapidly and simultaneously in focal regions of ischaemic injury in such a way as to suggest that the responses could be coordinated. The ability of neurons to modulate cerebral blood flow in regions of activation results from neurovascular coupling. But little is known about the microvessel-to-neuron direction of the relationship. The presence and participation of intervening glial cells implies the association of microvessels, glia, and neurons in a 'neurovascular unit'. The interdependent functions of the cellular and matrix components of this theoretical unit have not been rigorously explored, except under conditions of injury where, for the most part, only single components or tissue samples have been studied. Whereas maintenance or timely re-establishment of flow reduces tissue and neuron injury in both humans and animal models, protection of neuron function in humans has not prevented the evolution of injury despite the inherent mechanisms of neurovascular coupling. However, occlusion of flow to the brain rapidly identifies regions of neuron-vascular vulnerability within the vascular territory-at-risk. These coalesce to become the mature ischaemic lesion. The failure, so far, of clinical trials of neuron protectant agents to achieve detectable tissue salvage could be explained by the vulnerability (and lack of protection) of essential components of the 'unit'. This presentation summarizes evidence and thoughts on this topic. These support the need to understand component interactions within the neurovascular unit.
Conflict of interest statement
Conflict of interest statement: No conflict of interest was declared.
Figures
Fig. 1
A schematic of the ‘neurovascular unit’. Denoting the inter-relationship of microvessels to their dependent neurons and axons, via astrocytes. Injury to any component is likely to affect the function of the entire unit.
Fig. 2
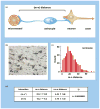
The effects of focal ischaemia on the neurovascular unit. (a) The distance between microvessels and neurons [(m–n) distance] within the striatum of the nonhuman primate (Papio anubis/cynocephalus) as a measure of neurovascular integrity. (b) Injured neurons (n*), uninjured neurons (n), and microvessels (m) scattered within the ischaemic core at 2 h following middle cerebral artery (MCA) occlusion in the nonhuman primate. (c) (m–n) Distance distribution based upon measurements of normoxic striatal neurons and their proximate microvessels. (d) Demonstration that neurons more distant from their nearest microvessel [(m–n*)] are significantly more likely to display injury than those at lesser distance [(m–n)] in the ischaemic striatum at 2 h post-MCA occlusion [24].
Fig. 3
Impact of ischaemia on the expression of matrix integrin receptors by microvessel endothelium, and matrix integrins and dystroglycan receptors on astrocyte end-feet in a model cerebral capillary. All changes occur within 2 h of MCA occlusion in the nonhuman primate striatum.
Similar articles
- Relationship of neurovascular elements to neuron injury during ischemia.
del Zoppo GJ. del Zoppo GJ. Cerebrovasc Dis. 2009;27 Suppl 1(Suppl 1):65-76. doi: 10.1159/000200442. Epub 2009 Apr 3. Cerebrovasc Dis. 2009. PMID: 19342834 Free PMC article. Review. - Toward the neurovascular unit. A journey in clinical translation: 2012 Thomas Willis Lecture.
Del Zoppo GJ. Del Zoppo GJ. Stroke. 2013 Jan;44(1):263-9. doi: 10.1161/STROKEAHA.112.653618. Epub 2012 Oct 2. Stroke. 2013. PMID: 23033344 - Microthrombus-Targeting Micelles for Neurovascular Remodeling and Enhanced Microcirculatory Perfusion in Acute Ischemic Stroke.
Lu Y, Li C, Chen Q, Liu P, Guo Q, Zhang Y, Chen X, Zhang Y, Zhou W, Liang D, Zhang Y, Sun T, Lu W, Jiang C. Lu Y, et al. Adv Mater. 2019 May;31(21):e1808361. doi: 10.1002/adma.201808361. Epub 2019 Apr 8. Adv Mater. 2019. PMID: 30957932 - Inflammation and the neurovascular unit in the setting of focal cerebral ischemia.
del Zoppo GJ. del Zoppo GJ. Neuroscience. 2009 Feb 6;158(3):972-82. doi: 10.1016/j.neuroscience.2008.08.028. Epub 2008 Aug 27. Neuroscience. 2009. PMID: 18824084 Free PMC article. Review. - Vascular aspects of neuroprotection.
Takahashi M, Macdonald RL. Takahashi M, et al. Neurol Res. 2004 Dec;26(8):862-9. doi: 10.1179/016164104X3815. Neurol Res. 2004. PMID: 15727270 Review.
Cited by
- Galectin-1 Contributes to Vascular Remodeling and Blood Flow Recovery After Cerebral Ischemia in Mice.
Cheng YH, Jiang YF, Qin C, Shang K, Yuan Y, Wei XJ, Xu Z, Luo X, Wang W, Qu WS. Cheng YH, et al. Transl Stroke Res. 2022 Feb;13(1):160-170. doi: 10.1007/s12975-021-00913-5. Epub 2021 May 10. Transl Stroke Res. 2022. PMID: 33973144 - Neurodegeneration in the pathogenesis of diabetic retinopathy: molecular mechanisms and therapeutic implications.
Stem MS, Gardner TW. Stem MS, et al. Curr Med Chem. 2013;20(26):3241-50. doi: 10.2174/09298673113209990027. Curr Med Chem. 2013. PMID: 23745549 Free PMC article. Review. - Pericyte hypoxia-inducible factor-1 (HIF-1) drives blood-brain barrier disruption and impacts acute ischemic stroke outcome.
Tsao CC, Baumann J, Huang SF, Kindler D, Schroeter A, Kachappilly N, Gassmann M, Rudin M, Ogunshola OO. Tsao CC, et al. Angiogenesis. 2021 Nov;24(4):823-842. doi: 10.1007/s10456-021-09796-4. Epub 2021 May 27. Angiogenesis. 2021. PMID: 34046769 Free PMC article. - Differential Effects of Pioglitazone in the Hippocampal CA1 Region Following Transient Forebrain Ischemia in Low- and High-Fat Diet-Fed Gerbils.
Moon SM, Choi GM, Yoo DY, Jung HY, Yim HS, Kim DW, Hwang IK, Cho BM, Chang IB, Cho SM, Won MH. Moon SM, et al. Neurochem Res. 2015 May;40(5):1063-73. doi: 10.1007/s11064-015-1568-3. Epub 2015 Apr 19. Neurochem Res. 2015. PMID: 25894680 - Modulation of microglial polarization by sequential targeting surface-engineered exosomes improves therapy for ischemic stroke.
Liu X, Hao Y, Huang Z, Shi Y, Su C, Zhao L. Liu X, et al. Drug Deliv Transl Res. 2024 Feb;14(2):418-432. doi: 10.1007/s13346-023-01408-6. Epub 2023 Aug 16. Drug Deliv Transl Res. 2024. PMID: 37587291
References
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–60. - PubMed
- Zonta M, Angulo M, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. - PubMed
- Nedergaard M, Ransom BR, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–30. - PubMed
- Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–9. - PubMed
- del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS053716/NS/NINDS NIH HHS/United States
- R01 NS053716-05/NS/NINDS NIH HHS/United States
- R37 NS038710/NS/NINDS NIH HHS/United States
- R37 NS038710-10/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical