Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences - PubMed (original) (raw)
Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences
M R Hutchinson et al. Neuroscience. 2010.
Abstract
Opioid-induced glial activation and its proinflammatory consequences have been associated with both reduced acute opioid analgesia and the enhanced development of tolerance, hyperalgesia and allodynia following chronic opioid administration. Intriguingly, recent evidence demonstrates that these effects can result independently from the activation of classical, stereoselective opioid receptors. Here, a structurally disparate range of opioids cause activation of signaling by the innate immune receptor toll like receptor 4 (TLR4), resulting in proinflammatory glial activation. In the present series of studies, we demonstrate that the (+)-isomers of methadone and morphine, which bind with negligible affinity to classical opioid receptors, induced upregulation of proinflammatory cytokine and chemokine production in rat isolated dorsal spinal cord. Chronic intrathecal (+)-methadone produced hyperalgesia and allodynia, which were associated with significantly increased spinal glial activation (TLR4 mRNA and protein) and the expression of multiple chemokines and cytokines. Statistical analysis suggests that a cluster of cytokines and chemokines may contribute to these nociceptive behavioral changes. Acute intrathecal (+)-methadone and (+)-morphine were also found to induce microglial, interleukin-1 and TLR4/myeloid differentiation factor-2 (MD-2) dependent enhancement of pain responsivity. In silico docking analysis demonstrated (+)-naloxone sensitive docking of (+)-methadone and (+)-morphine to human MD-2. Collectively, these data provide the first evidence of the pro-nociceptive consequences of small molecule xenobiotic activation of spinal TLR4 signaling independent of classical opioid receptor involvement.
Copyright 2010 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
Figure 1. Non-classical opioid induction of proinflammatory cytokines and chemokines by (+)-morphine and (+)-methadone from lumbar dorsal spinal cord in vitro
(+)-Morphine and (+)-methadone induce significant increases in proinflammatory cytokine and chemokine release were quantified in the supernatants from lumbar dorsal spinal cord sections incubated for 180 min in vitro with 100 µM of each drug compared to media alone (* P < 0.05). n=6 per treatment.
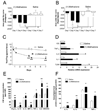
Figure 2. Chronic intrathecal administration of opioid-inactive (+)-methadone causes significant hyperalgesia and allodynia and associated glial activation and elevated proinflammatory mediator transcription and translation
Animals were administered once daily 15 µg (+)-methadone or saline (in 1 µl with a 25 µl flush) intrathecally for 7 days via an indwelling catheter. On days 1, 4 and 7 animals were tested for short baseline latency Hargreaves stimuli (A) to assess the presence of any analgesia and long baseline latency Hargreaves stimuli (B) to assess the development of hyperalgesia over a 2 h time course following drug administration. Panels A and B display the area under the analgesia time course on day 1, day 4 and day 7 for (+)-methadone (black bars) and saline (open bars). No significant analgesia resulted from the administration of the opioid inactive (+)-methadone. Instead significant reductions in withdrawal latencies developed across the treatment demonstration hyperalgesia on both short (A) and long (B) baseline latency stimuli Hargreaves tests. Mechanical allodynia (C) was assessed using von Frey filaments 60 min prior and 2 h after administration on day 1 and day 4 and only 60 min prior to drug administration on day 7 for (+)-methadone (⬆) or saline (✦). Tissues from these animals were collected for analysis in Panels D, E and F. D: Relative mRNA expression in lumbar dorsal spinal cord for TLR4, IL-1, IL-6 and TNF-α and IL-1 converting enzyme (ICE) following 7 days intrathecal (+)-methadone (black bar) or saline (open bar) administration. E: CSF protein levels following 7 days intrathecal (+)-methadone (black bar) or saline (open bar) administration. F: Lumbar dorsal spinal cord protein levels following 7 days intrathecal (+)-methadone (black bar) or saline (open bar) administration. * P < 0.05; ** P < 0.01; *** P < 0.001. n = 6 per group
Figure 3. Intrathecal interleukin-1 receptor antagonist "unmasks" κ opioid analgesia following acute intrathecal (+)-methadone administration which is dependent on microglial and TLR4 activation
A: Intrathecal injection of 15 µg of (+)-methadone (in 1 µl with a 10 µl flush; ⬆) produces no significant analgesia (5 min to 95 min compared to vehicle treated animals or saline plus IL-1ra treated animals; O; P > 0.05). However, following intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; ⬆) significant analgesia is unmasked (115 min to 165 min; P < 0.05). B: Intrathecal co-administration of 15 µg of (+)-methadone (15 µg (+)-methadone in 1 µl) and IL-1ra (100 µg in 1 µl with 10 µl flush; ⬆) produced no significant analgesia, even following a further intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; ⬆). C: Intrathecal co-administration of 15 µg of (+)-methadone (15 µg (+)-methadone in 1 µl) and minocycline (a microglial activation attenuator; 100 µg in 3 µl at time of catheter implant and then 33.3 µg in 1 and with 10 µl flush at time 0; ⬆) produced no significant analgesia, even following a further intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; ⬆). D: Intrathecal co-administration of 15 µg of (+)-methadone (15 µg (+)-methadone in 1 µl) and (+)-naloxone (a novel TLR4 signaling inhibitor; 60 µg in 1 µl at with 10 µl flush; ⬆) produced no significant analgesia, even following a further intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; ⬆). E: Intrathecal injection of 15 µg of (+)-methadone (in 1 µl with a 10 µl flush; ⬆) produces no significant analgesia. Following intrathecal co-administration of IL-1ra and (−)-naloxone (a non-specific opioid receptor antagonist; 100 µg IL-1ra in 1 µl and 20 µg (−)-naloxone in 1 µl with a 10 µl flush; ⬆) no further analgesia was unmasked. F: Intrathecal injection of 15 µg of (+)-methadone (in 1 µl with a 10 µl flush; ⬆) produces no significant analgesia. Following intrathecal co-administration of IL-1ra and naloxonazine (a specific µ1 opioid receptor antagonist; 100 µg IL-1ra in 1 µl and 5 µg naloxonazine in 1 µl with a 10 µl flush; ⬆) significant analgesia is unmasked (115 min to 165 min; P < 0.05). G: Intrathecal injection of 15 µg of (+)-methadone (in 1 µl with a 10 µl flush; ⬆) produces no significant analgesia. Following intrathecal co-administration of IL-1ra and naltrindole (a specific δ opioid receptor antagonist; 100 µg IL-1ra in 1 µl and 5 µg naltrindole in 1 µl with a 10 µl flush; ⬆) significant analgesia is unmasked (115 min to 165 min; P < 0.05). F: Intrathecal injection of 15 µg of (+)-methadone (in 1 µl with a 10 µl flush; ⬆) produces no significant analgesia. Following intrathecal co-administration of IL-1ra and norBNI (a specific κ opioid receptor antagonist; 100 µg IL-1ra in 1 µl and 5 µg norBNI in 1 µl with a 10 µl flush; ⬆) no significant analgesia is unmasked. n = 6 per group.
Figure 4. Intrathecal (+)-morphine induces interleukin-1 induced hyperalgesia which is dependent on microglial and TLR4 activation
A: Intrathecal injection of 15 µg of (+)-morphine (in 1 µl with a 10 µl flush; ■) produces significant hyperalgesia (15 min to 55 min compared to vehicle treated animals O or saline plus IL-1ra treated animals; P > 0.05). This hyperalgesia is not affected by subsequent intrathecal saline administration (65 min to 125 min; P < 0.05). B: Intrathecal injection of 15 µg of (+)-morphine (in 1 µl with a 10 µl flush; ■) produces significant hyperalgesia (25 min to 55 min compared to vehicle treated animals O or saline plus IL-1ra treated animals; _P_ > 0.05). However, following intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; ■) significant reversal of the hyperalgesia occurs (65 min to 125 min; P > 0.05). C: Intrathecal co-administration of 15 µg of (+)-morphine (15 µg (+)-morphine in 1 µl) and IL-1ra (100 µg in 1 µl with 10 µl flush; ■) produced no significant hyperalgesia, with no significant effect of a further intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; ■). D: Intrathecal co-administration of 15 µg of (+)-morphine (15 µg (+)-morphine in 1 µl) and minocycline (a microglial activation attenuator; 100 µg in 3 µl at time of catheter implant and then 33.3 µg in 1 and with 10 µl flush at time 0; ■) produced no significant hyperalgesia, with no significant effect following a further intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; ■). E: Intrathecal co-administration of 15 µg of (+)-morphine (15 µg (+)-morphine in 1 µl) and (+)-naloxone (a novel TLR4 signaling inhibitor; 60 µg 1 µl and with 10 µl flush; ■) produced no significant hyperalgesia, with no significant effect following a further intrathecal IL-1ra (100 µg in 1 µl with a 10 µl flush; ■). n = 6 per group
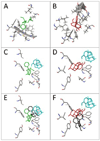
Figure 5. (+)-Methadone and (+)-morphine dock in silico to human MD-2 in a (+)-naloxone sensitive fashion
The optimal docking conformations for (+)-methadone (panel A; green) and (+)-morphine (panel B; red) alone are shown following docking in silico to human MD-2 with the MD-2 interaction residues displayed. The prior in silico docking of (+)-naloxone (turquoise) to MD-2 substantially modified (+)-methadone (panel C; green) and (+)-morphine (panel D; red) preferred docking conformation when compared to their docking conformations alone (panels E and F, conformations of docking to MD-2 alone shown in black).
Similar articles
- Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta.
Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, Watkins LR. Lewis SS, et al. Neuroscience. 2010 Jan 20;165(2):569-83. doi: 10.1016/j.neuroscience.2009.10.011. Neuroscience. 2010. PMID: 19833175 Free PMC article. - Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia.
Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR. Hutchinson MR, et al. Brain Behav Immun. 2008 Nov;22(8):1178-89. doi: 10.1016/j.bbi.2008.05.004. Epub 2008 Jul 2. Brain Behav Immun. 2008. PMID: 18599265 Free PMC article. - Involvement of TCF7L2 in generation of morphine-induced antinociceptive tolerance and hyperalgesia by modulating TLR4/ NF-κB/NLRP3 in microglia.
Chen J, Wang G, Sun T, Ma C, Huo X, Kong Y. Chen J, et al. Toxicol Appl Pharmacol. 2021 Apr 1;416:115458. doi: 10.1016/j.taap.2021.115458. Epub 2021 Feb 17. Toxicol Appl Pharmacol. 2021. PMID: 33607128 - Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility.
Zhang P, Yang M, Chen C, Liu L, Wei X, Zeng S. Zhang P, et al. Front Immunol. 2020 Jul 8;11:1455. doi: 10.3389/fimmu.2020.01455. eCollection 2020. Front Immunol. 2020. PMID: 32733481 Free PMC article. Review. - Inflammatory mediators of opioid tolerance: Implications for dependency and addiction.
Eidson LN, Murphy AZ. Eidson LN, et al. Peptides. 2019 May;115:51-58. doi: 10.1016/j.peptides.2019.01.003. Epub 2019 Mar 16. Peptides. 2019. PMID: 30890355 Free PMC article. Review.
Cited by
- Lack of Specific Involvement of (+)-Naloxone and (+)-Naltrexone on the Reinforcing and Neurochemical Effects of Cocaine and Opioids.
Tanda G, Mereu M, Hiranita T, Quarterman JC, Coggiano M, Katz JL. Tanda G, et al. Neuropsychopharmacology. 2016 Oct;41(11):2772-81. doi: 10.1038/npp.2016.91. Epub 2016 Jun 14. Neuropsychopharmacology. 2016. PMID: 27296151 Free PMC article. - The PPARγ Agonist Pioglitazone Fails to Alter the Abuse Potential of Heroin, But Does Reduce Heroin Craving and Anxiety.
Jones JD, Bisaga A, Metz VE, Manubay JM, Mogali S, Ciccocioppo R, Madera G, Doernberg M, Comer SD. Jones JD, et al. J Psychoactive Drugs. 2018 Nov-Dec;50(5):390-401. doi: 10.1080/02791072.2018.1508789. Epub 2018 Sep 11. J Psychoactive Drugs. 2018. PMID: 30204554 Free PMC article. Clinical Trial. - Interactions of Opioids and HIV Infection in the Pathogenesis of Chronic Pain.
Liu B, Liu X, Tang SJ. Liu B, et al. Front Microbiol. 2016 Feb 10;7:103. doi: 10.3389/fmicb.2016.00103. eCollection 2016. Front Microbiol. 2016. PMID: 26903982 Free PMC article. Review. - DAT isn't all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling.
Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomrenze MB, Galer EL, Kopajtic TA, Li CM, Amat J, Larson G, Cooper DC, Huang Y, O'Neill CE, Yin H, Zahniser NR, Katz JL, Rice KC, Maier SF, Bachtell RK, Watkins LR. Northcutt AL, et al. Mol Psychiatry. 2015 Dec;20(12):1525-37. doi: 10.1038/mp.2014.177. Epub 2015 Feb 3. Mol Psychiatry. 2015. PMID: 25644383 Free PMC article. - Microglia in neuroimmunopharmacology and drug addiction.
Li H, Watkins LR, Wang X. Li H, et al. Mol Psychiatry. 2024 Jun;29(6):1912-1924. doi: 10.1038/s41380-024-02443-6. Epub 2024 Feb 2. Mol Psychiatry. 2024. PMID: 38302560 Review.
References
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. - PubMed
- Carmody J. Avoiding fallacies in nociceptive measurements. Pain. 1995;63:136. - PubMed
- Chao CC, Gekker G, Sheng WS, Hu S, Tsang M, Peterson PK. Priming effect of morphine on the production of tumor necrosis factor-alpha by microglia: implications in respiratory burst activity and human immunodeficiency virus-1 expression. J Pharmacol Exp Ther. 1994;269:198–203. - PubMed
- Casy AF. The Steric Factor in Medicinal Chemistry: Dissymmetric Probes of Pharmacological Receptors. Oxford: Plenum Press; 1993.
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA024044/DA/NIDA NIH HHS/United States
- K05 DA024044-01A1/DA/NIDA NIH HHS/United States
- R01 DE017782-01/DE/NIDCR NIH HHS/United States
- K02 DA015642-05/DA/NIDA NIH HHS/United States
- DA015642/DA/NIDA NIH HHS/United States
- K02 DA015642/DA/NIDA NIH HHS/United States
- DA017670/DA/NIDA NIH HHS/United States
- K05 DA024044/DA/NIDA NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 DA017670-01A1/DA/NIDA NIH HHS/United States
- R01 DA017670/DA/NIDA NIH HHS/United States
- R01 DE017782/DE/NIDCR NIH HHS/United States
- DE017782/DE/NIDCR NIH HHS/United States
- R01 DA023132/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources