A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry - PubMed (original) (raw)
A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry
Mari Mino-Kenudson et al. Clin Cancer Res. 2010.
Abstract
Purpose: Approximately 5% of lung adenocarcinomas harbor an EML4-ALK gene fusion and define a unique tumor group that may be responsive to targeted therapy. However ALK-rearranged lung adenocarcinomas are difficult to detect by either standard fluorescence in situ hybridization or immunohistochemistry (IHC) assays. In the present study, we used novel antibodies to compare ALK protein expression in genetically defined lung cancers and anaplastic large cell lymphomas.
Experimental design: We analyzed 174 tumors with one standard and two novel monoclonal antibodies recognizing the ALK protein. Immunostained tissue sections were assessed for the level of tumor-specific ALK expression by objective quantitative image analysis and independently by three pathologists.
Results: ALK protein is invariably and exclusively expressed in ALK-rearranged lung adenocarcinomas but at much lower levels than in the prototypic ALK-rearranged tumor, anaplastic large cell lymphoma, and as a result, is often not detected by conventional IHC. We further validate a novel IHC that shows excellent sensitivity and specificity (100% and 99%, respectively) for the detection of ALK-rearranged lung adenocarcinomas in biopsy specimens, with excellent interobserver agreement between pathologists (kappa statistic, 0.94).
Conclusions: Low levels of ALK protein expression is a characteristic feature of ALK-rearranged lung adenocarcinomas. However, a novel, highly sensitive IHC assay reliably detects lung adenocarcinomas with ALK rearrangements and obviates the need for fluorescence in situ hybridization analysis for the majority of cases, and therefore could be routinely applicable in clinical practice to detect lung cancers that may be responsive to ALK inhibitors.
Figures
Figure 1
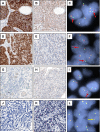
Immunohistochemical staining of FFPE tissues with D5F3 or D9E4 antibodies detect ALK protein expression in _ALK_-rearranged tumors. Cases of cytogenetically confirmed, _ALK_-rearranged anaplastic large cell lymphoma (A-C), _ALK_-germline anaplastic large cell lymphoma (D-F), and _ALK_-rearranged inflammatory myofibroblastic tumor (G-I) stained with antibodies D5F3 (A, D, G), D9E4 (B, E, H), and ALK1 (C, F, I).
Figure 2
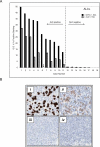
Correlation in staining between D5F3 and ALK1 antibodies for anaplastic large cell lymphoma. (A) Quantitative assessment of staining intensities for 19 tumors previously determined as ALK-positive or ALK-negative (by genetic testing and/or IHC) at the time of diagnosis (_black bars_= D5F3 antibody, _grey bars_= ALK1 antibody). (B) Photomicrographs of Case #11 (i; ii) and Case #12 (iii; iv) stained with D5F3 antibody (i; iii) and ALK1 antibody (ii; iv). _N.D._= not determined.
Figure 3
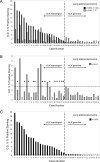
Correlation in staining between D5F3 and ALK1 antibodies in lung adenocarcinoma. Quantitative assessment of staining intensities for 37 tumors determined to be _ALK_-rearranged (Cases 1–22) or _ALK_-germline (Cases 23–37) by FISH analysis. (A) Comparison of staining intensities using D5F3 antibody (black bars) or ALK1 antibody (grey bars). (B) Staining intensities using ALK1 antibody with the maximal level of background staining observed among the _ALK_-germline tumors indicated (horizontal dotted line) and the cases of _ALK_-rearranged tumors that fail to show staining over this background level (asterisks). (C) Staining intensities using D5F3 antibody with the maximal level of background staining observed among the _ALK_-germline tumors indicated (horizontal dotted line).
Figure 4
Photomicrographs of lung adenocarcinomas stained with D5F3 and ALK1 antibodies and analyzed by FISH. (A, B, C) Case #1, (D, E, F) Case #2, (G, H, I) Case #22, and (J, K, L) Case #24 stained with D5F3 antibody (A, D, G, J), ALK1 antibody (B, E, H, K), or analyzed by FISH (C, F, I, L; _red arrow_= split red-green signals indicative of _ALK_-rearrangement, _yellow arrow_= touching red-green signals not indicative of _ALK_-rearrangement).
Figure 5
Multiple biopsy samples stained with D5F3 antibody from a patient with an _ALK_-rearranged lung adenocarcinoma. Two biopsies (A, B) show faint, but definitive cytoplasmic staining, one biopsy (C) shows equivocal staining, and one biopsy (D) shows no staining for ALK protein. The inset in D demonstrates split red-green signals indicative of ALK-rearrangement by FISH.
Similar articles
- ALK-rearranged lung cancer in Chinese: a comprehensive assessment of clinicopathology, IHC, FISH and RT-PCR.
Li Y, Pan Y, Wang R, Sun Y, Hu H, Shen X, Lu Y, Shen L, Zhu X, Chen H. Li Y, et al. PLoS One. 2013 Jul 26;8(7):e69016. doi: 10.1371/journal.pone.0069016. Print 2013. PLoS One. 2013. PMID: 23922677 Free PMC article. - A novel, highly sensitive ALK antibody 1A4 facilitates effective screening for ALK rearrangements in lung adenocarcinomas by standard immunohistochemistry.
Gruber K, Kohlhäufl M, Friedel G, Ott G, Kalla C. Gruber K, et al. J Thorac Oncol. 2015 Apr;10(4):713-6. doi: 10.1097/JTO.0000000000000427. J Thorac Oncol. 2015. PMID: 25789835 - Bright-field dual-color chromogenic in situ hybridization for diagnosing echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase-positive lung adenocarcinomas.
Yoshida A, Tsuta K, Nitta H, Hatanaka Y, Asamura H, Sekine I, Grogan TM, Fukayama M, Shibata T, Furuta K, Kohno T, Tsuda H. Yoshida A, et al. J Thorac Oncol. 2011 Oct;6(10):1677-86. doi: 10.1097/JTO.0b013e3182286d25. J Thorac Oncol. 2011. PMID: 21921848 - Biomarkers for ALK and ROS1 in Lung Cancer: Immunohistochemistry and Fluorescent In Situ Hybridization.
Luk PP, Selinger CI, Mahar A, Cooper WA. Luk PP, et al. Arch Pathol Lab Med. 2018 Aug;142(8):922-928. doi: 10.5858/arpa.2017-0502-RA. Epub 2018 Jun 14. Arch Pathol Lab Med. 2018. PMID: 29902067 Review.
Cited by
- Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: a success story to usher in the second decade of molecular targeted therapy in oncology.
Ou SH, Bartlett CH, Mino-Kenudson M, Cui J, Iafrate AJ. Ou SH, et al. Oncologist. 2012;17(11):1351-75. doi: 10.1634/theoncologist.2012-0311. Epub 2012 Sep 18. Oncologist. 2012. PMID: 22989574 Free PMC article. Review. - Therapeutic Advances of Rare ALK Fusions in Non-Small Cell Lung Cancer.
Xiang Y, Zhang S, Fang X, Jiang Y, Fang T, Liu J, Lu K. Xiang Y, et al. Curr Oncol. 2022 Oct 16;29(10):7816-7831. doi: 10.3390/curroncol29100618. Curr Oncol. 2022. PMID: 36290895 Free PMC article. Review. - Molecularly targeted approaches herald a new era of non-small-cell lung cancer treatment.
Kaneda H, Yoshida T, Okamoto I. Kaneda H, et al. Cancer Manag Res. 2013 Jun 7;5:91-101. doi: 10.2147/CMAR.S32973. Print 2013. Cancer Manag Res. 2013. PMID: 23785245 Free PMC article. - ALK-rearranged lung cancer in Chinese: a comprehensive assessment of clinicopathology, IHC, FISH and RT-PCR.
Li Y, Pan Y, Wang R, Sun Y, Hu H, Shen X, Lu Y, Shen L, Zhu X, Chen H. Li Y, et al. PLoS One. 2013 Jul 26;8(7):e69016. doi: 10.1371/journal.pone.0069016. Print 2013. PLoS One. 2013. PMID: 23922677 Free PMC article. - Discrepancies between ALK protein disruption and occurrence of ALK gene rearrangement in Polish NSCLC patients.
Grenda A, Jarosz B, Krawczyk P, Kucharczyk T, Wojas-Krawczyk K, Reszka K, Krukowska K, Nicoś M, Pankowski J, Bryl M, Ramlau R, Kuźnar-Kamińska B, Grodzki T, Szczęsna A, Siemiątkowska K, Szumiło J, Batura-Gabryel H, Palonka M, Milanowski J. Grenda A, et al. J Thorac Dis. 2018 Aug;10(8):4994-5009. doi: 10.21037/jtd.2018.07.28. J Thorac Dis. 2018. PMID: 30233874 Free PMC article.
References
- Alberg AJ, Ford JG, Samet JM. Chest. 2nd edition Vol. 132. 2007. Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines; pp. 29S–55S. - PubMed
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. - PubMed
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. - PubMed
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med. 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 CA090578/CA/NCI NIH HHS/United States
- CA090578/CA/NCI NIH HHS/United States
- R01CA136851/CA/NCI NIH HHS/United States
- R01 CA136851-01A1/CA/NCI NIH HHS/United States
- P50 CA090578/CA/NCI NIH HHS/United States
- R01 CA114465/CA/NCI NIH HHS/United States
- R01 CA136851/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical