Whisker-related axonal patterns and plasticity of layer 2/3 neurons in the mouse barrel cortex - PubMed (original) (raw)
Whisker-related axonal patterns and plasticity of layer 2/3 neurons in the mouse barrel cortex
Keisuke Sehara et al. J Neurosci. 2010.
Abstract
Elucidating neuronal circuits and their plasticity in the cerebral cortex is one of the important questions in neuroscience research. Here we report novel axonal trajectories and their plasticity in the mouse somatosensory barrel cortex. We selectively visualized layer 2/3 neurons using in utero electroporation and examined the axonal trajectories of layer 2/3 neurons. We found that the axons of layer 2/3 neurons preferentially run in the septal regions of layer 4 and named this axonal pattern "barrel nets." The intensity of green fluorescent protein in the septal regions was markedly higher compared with that in barrel hollows. Focal in utero electroporation revealed that the axons in barrel nets were indeed derived from layer 2/3 neurons in the barrel cortex. During development, barrel nets became visible at postnatal day 10, which was well after the initial appearance of barrels. When whisker follicles were cauterized within 3 d after birth, the whisker-related pattern of barrel nets was altered, suggesting that cauterization of whisker follicles results in developmental plasticity of barrel nets. Our results uncover the novel axonal trajectories of layer 2/3 neurons with whisker-related patterns and their developmental plasticity in the mouse somatosensory cortex. Barrel nets should be useful for investigating the pattern formation and axonal reorganization of intracortical neuronal circuits.
Figures
Figure 1.
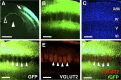
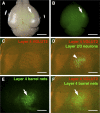
Axonal organization of layer 2/3 neurons in the barrel cortex. GFP was expressed in layer 2/3 neurons using in utero electroporation at E15.5, and 50 μm coronal sections were made. A, GFP fluorescence in coronal sections prepared at P15. Arrow, Barrel cortex; closed arrowhead, hippocampus; open arrowhead, callosal fibers. Scale bar, 1 mm. B, C, Higher-magnification images of GFP-positive structures (B) and Hoechst 33342 staining (C) in the barrel cortex. Scale bars, 250 μm. D–F, Coronal sections prepared at P9 were immunostained with anti-VGLUT2 antibody. GFP-positive structures (D), VGLUT2-positive TCAs (E), and a merged image (F) are shown. Note the high levels of GFP fluorescence (arrowheads) in the regions between TCA patches in layer 4. Scale bars, 200 μm.
Figure 2.
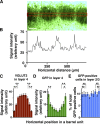
Quantification of GFP fluorescence in layer 4 and GFP-positive cells in layer 2/3 of the barrel cortex. GFP was expressed in layer 2/3 neurons using in utero electroporation at E15.5, and 50 μm coronal sections were made at P15. A, GFP fluorescence intensities were measured within a rectangular area (red box) in layer 4. Scale bar, 100 μm. B, GFP fluorescence intensities plotted against the horizontal distance. The background fluorescence intensity was subtracted from the measured fluorescence intensities. The horizontal axis represents the distance from the left end of A. C–E, The distribution patterns of VGLUT2 signal intensities (C), GFP intensities (D), and the number of GFP-positive cells (E) within barrel units. Here, a “barrel unit” refers to a region containing one barrel and its septal rim. Normalized VGLUT2 signal intensities in layer 4 exhibited a center-high, periphery-low manner (C), which supports the validity of barrel unit extraction. Note that GFP intensities in layer 4 are significantly higher in the peripheral regions of a barrel unit compared with those in the central region (D), while no significant difference was detected in the case of the number of GFP-positive cells in layer 2/3 (E). Bars represent mean ± SEM (n = 16 barrel units). *p < 0.05; **_p_ < 0.01; NS, _p_ > 0.2, two-sided Wilcoxon's signed rank test.
Figure 3.
Distribution patterns of GFP signals in tangential sections of the barrel cortex. GFP was expressed in layer 2/3 neurons using in utero electroporation, and 50 μm cortical tangential sections were prepared at P15. The sections corresponding to layer 4 were used for further analyses. A, A low-magnification image of the entire tangential section. Note that whisker-related patterns are visible in the barrel cortex, whereas surrounding areas do not show apparent patterns. Arrowhead, Injection site of plasmid; asterisk, olfactory bulb. Scale bar, 2 mm. B, C, Higher-magnification images of the barrel cortex. Scale bars, 200 μm. D–F, Tangential sections were immunostained with anti-VGLUT2 antibody. GFP-positive axons (D), VGLUT2-positive TCAs (E), and a merged image (F) are shown. Note that GFP signals are predominantly located in the septal regions (arrows). Scale bars, 200 μm.
Figure 4.
Distribution patterns of GFP signals examined using confocal microscopy. GFP was expressed in layer 2/3 neurons using in utero electroporation, and sections prepared at P15 were examined with confocal microscopy. A, Tangential sections were stained with anti-VGLUT2 antibody. The _Z_-stack images were three-dimensionally reconstructed, with green representing the maximum projection image of GFP and red representing the surface image of VGLUT2 immunoreactivity. Scale bars, 50 μm in each direction. B, A high-magnification confocal image of a tangential section stained with anti-VGLUT2 antibody. Note that GFP signals are predominantly found in septa (arrow) compared with barrel hollows (bh). GFP signals are also found in some parts of barrel walls (closed arrowhead), but not in other parts of barrel walls (open arrowhead). Scale bar, 25 μm. C, D, High-magnification confocal images of GFP-positive axons in coronal sections. The _Z_-stack images were three-dimensionally reconstructed and displayed as maximum projection images. GFP-positive structures in the septum (C) and in the barrel hollow (D) are shown. Scale bars, 10 μm.
Figure 5.
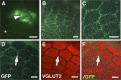
Selective expression of GFP within the barrel cortex using focal in utero electroporation. In utero electroporation was performed at E15.5 with small electrodes of 1 mm diameter, and the brain was dissected at P15. A, A macroscopic image of the brain. A bright-field image and a GFP fluorescence image are merged. GFP signal (arrow) was observed in a small region. Scale bar, 3 mm. B, A single-channel image of GFP fluorescence from A is shown. Arrow indicates a GFP-positive area. Scale bar, 3 mm. C–F, The cortical hemisphere of the electroporated side was flattened, and tangential sections of 50 μm thickness were made. The section containing layer 4 was stained with anti-VGLUT2 antibody. VGLUT2-positive TCAs (C), GFP-positive axons (E), and a merged image (F) are shown. In D, the single-channel image of VGLUT2 staining was also overlaid with a GFP fluorescence image from another section, which contained GFP-positive layer 2/3 neurons, using blood vessels as landmarks. Note that barrel nets are clearly visible (arrows) even when the area containing GFP-positive cells is restricted to the barrel field (arrowhead). Scale bars, 1 mm.
Figure 6.
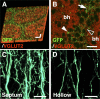
Distribution patterns of synaptophysin-EGFP expressed in layer 2/3 neurons. Using in utero electroporation, synaptophysin-EGFP (Sph-EGFP) and mCherry were coexpressed in layer 2/3 neurons in the barrel cortex. Coronal sections of 50 μm thickness were prepared at P15. A, A low-magnification image of the barrel cortex. Barrel nets were visualized by mCherry (arrowheads). Scale bar, 200 μm. B, C, High-magnification images of the regions shown in A. Note a number of synaptophysin-EGFP puncta (arrows) observed in barrel nets (B) as well as in layer 5 (C). Scale bars, 20 μm.
Figure 7.
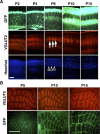
The formation of barrel nets during development. A, Coronal sections of the barrel cortex were prepared at the indicated ages and stained with anti-VGLUT2 antibody and Hoechst 33342. Note that whisker-related patterns of TCAs (arrows) and cytoarchitectonic barrels (open arrowheads) are visible at P6, whereas GFP-positive barrel nets become detectable at P10 (closed arrowheads). Scale bar, 200 μm. B, Tangential sections prepared at indicated ages were stained with anti-VGLUT2 antibody. Note that whisker-related patterns of TCAs are present at P5, while GFP-positive barrel nets are visible only at P10 and P15. Scale bar, 500 μm.
Figure 8.
The effect of follicle cauterization on whisker-related patterns of barrel nets during development. A, The experimental procedure for examining the effect of follicle cauterization. In utero electroporation was performed at E15.5, and follicles of row C whiskers were cauterized at one of the indicated time points. Tangential sections of the cerebral cortex were prepared at P15 and stained with anti-VGLUT2 antibody. B, Distribution patterns of VGLUT2 immunoreactivity and GFP signals in tangential sections. Follicle cauterization at P1 or P3 resulted in reorganization of the barrels corresponding to row C (arrowheads), and whisker-related patterns of barrel nets were altered accordingly (arrows). Whisker-related patterns of barrels and barrel nets were not altered by follicle cauterization at P6. Scale bar, 200 μm.
Similar articles
- Neuronal circuits with whisker-related patterns.
Sehara K, Kawasaki H. Sehara K, et al. Mol Neurobiol. 2011 Jun;43(3):155-62. doi: 10.1007/s12035-011-8170-8. Epub 2011 Mar 3. Mol Neurobiol. 2011. PMID: 21365361 Review. - Distinct developmental principles underlie the formation of ipsilateral and contralateral whisker-related axonal patterns of layer 2/3 neurons in the barrel cortex.
Sehara K, Wakimoto M, Ako R, Kawasaki H. Sehara K, et al. Neuroscience. 2012 Dec 13;226:289-304. doi: 10.1016/j.neuroscience.2012.09.010. Epub 2012 Sep 19. Neuroscience. 2012. PMID: 23000626 - Classic Cadherins Mediate Selective Intracortical Circuit Formation in the Mouse Neocortex.
Wakimoto M, Sehara K, Ebisu H, Hoshiba Y, Tsunoda S, Ichikawa Y, Kawasaki H. Wakimoto M, et al. Cereb Cortex. 2015 Oct;25(10):3535-46. doi: 10.1093/cercor/bhu197. Epub 2014 Sep 17. Cereb Cortex. 2015. PMID: 25230944 - Metabolic barrel representations with various patterns of neonatal whisker deafferentation in rats.
Shin JW, Lee DJ, Jung HS, Sohn NW. Shin JW, et al. Int J Dev Neurosci. 2005 Oct;23(6):537-44. doi: 10.1016/j.ijdevneu.2005.05.006. Int J Dev Neurosci. 2005. PMID: 15963678 - Synaptic biology of barrel cortex circuit assembly.
Vitali I, Jabaudon D. Vitali I, et al. Semin Cell Dev Biol. 2014 Nov;35:156-64. doi: 10.1016/j.semcdb.2014.07.009. Epub 2014 Jul 28. Semin Cell Dev Biol. 2014. PMID: 25080022 Review.
Cited by
- Microglia contact induces synapse formation in developing somatosensory cortex.
Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J. Miyamoto A, et al. Nat Commun. 2016 Aug 25;7:12540. doi: 10.1038/ncomms12540. Nat Commun. 2016. PMID: 27558646 Free PMC article. - Neuronal circuits with whisker-related patterns.
Sehara K, Kawasaki H. Sehara K, et al. Mol Neurobiol. 2011 Jun;43(3):155-62. doi: 10.1007/s12035-011-8170-8. Epub 2011 Mar 3. Mol Neurobiol. 2011. PMID: 21365361 Review. - Stress-activated protein kinase MKK7 regulates axon elongation in the developing cerebral cortex.
Yamasaki T, Kawasaki H, Arakawa S, Shimizu K, Shimizu S, Reiner O, Okano H, Nishina S, Azuma N, Penninger JM, Katada T, Nishina H. Yamasaki T, et al. J Neurosci. 2011 Nov 16;31(46):16872-83. doi: 10.1523/JNEUROSCI.1111-11.2011. J Neurosci. 2011. PMID: 22090513 Free PMC article. - Sensory input drives rapid homeostatic scaling of the axon initial segment in mouse barrel cortex.
Jamann N, Dannehl D, Lehmann N, Wagener R, Thielemann C, Schultz C, Staiger J, Kole MHP, Engelhardt M. Jamann N, et al. Nat Commun. 2021 Jan 4;12(1):23. doi: 10.1038/s41467-020-20232-x. Nat Commun. 2021. PMID: 33397944 Free PMC article. - Gyrification of the cerebral cortex requires FGF signaling in the mammalian brain.
Matsumoto N, Shinmyo Y, Ichikawa Y, Kawasaki H. Matsumoto N, et al. Elife. 2017 Nov 14;6:e29285. doi: 10.7554/eLife.29285. Elife. 2017. PMID: 29132503 Free PMC article.
References
- Alloway KD. Information processing streams in rodent barrel cortex: the differential functions of barrel and septal circuits. Cereb Cortex. 2008;18:979–989. - PubMed
- Alloway KD, Zhang M, Chakrabarti S. Septal columns in rodent barrel cortex: functional circuits for modulating whisking behavior. J Comp Neurol. 2004;480:299–309. - PubMed
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
- Barnett MW, Watson RF, Kind PC. Pathways to barrel development. In: Erzurumlu RS, Guido W, Molnar Z, editors. Development and plasticity in sensory thalamus and cortex. New York: Springer; 2006. pp. 138–157.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources