Notch signaling influences neuroprotective and proliferative properties of mature Müller glia - PubMed (original) (raw)
Notch signaling influences neuroprotective and proliferative properties of mature Müller glia
Kanika Ghai et al. J Neurosci. 2010.
Abstract
Notch signaling is known to play important roles during retinal development. Recently, Notch signaling has been shown to be active in proliferating Müller glia in acutely damaged chick retina (Hayes et al., 2007). However, the roles of Notch in mature, undamaged retina remain unknown. Thus, the purpose of this study was to determine the role of the Notch-signaling pathway in the postnatal retina. Here we show that components of the Notch-signaling pathway are expressed in most Müller glia at low levels in undamaged retina. The expression of Notch-related genes varies during early postnatal development and across regions, with higher expression in peripheral versus central retina. Blockade of Notch activity with a small molecule inhibitor before damage was protective to retinal interneurons (amacrine and bipolar cells) and projection neurons (ganglion cells). In the absence of damage, Notch is upregulated in retinas treated with insulin and FGF2; the combination of these factors is known to stimulate the proliferation and dedifferentiation of Müller glia (Fischer et al., 2002b). Inhibition of Notch signaling during FGF2 treatment reduces levels of the downstream effectors of the MAPK-signaling pathway-p38 MAPK and pCREB in Müller glia. Further, inhibition of Notch activity potently inhibits FGF2-induced proliferation of Müller glia. Together, our data indicate that Notch signaling is downstream of, and is required for, FGF2/MAPK signaling to drive the proliferation of Müller glia. In addition, our data suggest that low levels of Notch signaling in Müller glia diminish the neuroprotective activities of these glial cells.
Figures
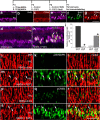
Figure 1.
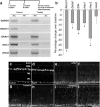
Components of the Notch-signaling pathway are expressed at low levels in the postnatal chicken retina. Vertical sections of the P7 retina were hybridized with riboprobes to cNotch1 (a, b; blue) and labeled for the glial marker 2M6 (c; red). d is a merged image of b and c. e–h are sevenfold enlargements of corresponding boxed areas in d. Arrows in b–h indicate cNotch1 and 2M6 double-positive cells. The scale bar (50 μm) in a applies to a alone, and that in b applies to b–d. mRNA was harvested from retinas, reverse transcribed to generate cDNA, and amplified using PCR (i) or real-time PCR (j–l). The PCR products were run on an agarose gel, and stained with ethidium bromide (i). Reactions minus the reverse transcriptase enzyme (RT−) were used as a negative control for each primer set. The percentage change in cDNA levels for cNotch1 (j), cHes1 (k), and cHes5 (l) were calculated from C(t) values that were normalized to GAPDH. Relative expression levels were measured for each mRNA at P0 in central retina (set as baseline) and at P7, P14, and P21 in central and peripheral retina and E3 whole retina. Significance of difference (*p < 0.005 between two time points within the same region, #p < 0.01 between central and peripheral retina at the same time point) was determined by using a two-factor ANOVA followed by a post hoc t test. RT, Reverse transcriptase; INL, inner nuclear layer; IPL, inner plexiform layer.
Figure 2.
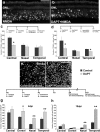
Inhibition of Notch signaling reduces the expression of components of the Notch-signaling pathway in undamaged chicken retina. mRNA was harvested from central and peripheral retinas obtained from eyes injected with vehicle (Control) or DAPT (Treated). mRNA was reverse transcribed to generate cDNA and amplified using PCR (a) or real-time PCR (b). The PCR products were run on an agarose gel and stained with ethidium bromide (a). For real-time PCR, C(t) values were normalized to GAPDH and the fold difference between control and treated samples was determined using the ΔCt method and represented as a percentage change from baseline (b). c–j, Representative images from retinas harvested 24 h after the last dose of vehicle or DAPT. Vertical sections of the P7 retina were labeled for Sox9 (c, d), GFAP (e, f), transitin (g, h), or vimentin (i, j). RT, Reverse transcriptase; ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer. The scale bar (50 μm) in j applies to c–j.
Figure 3.
Inhibition of Notch signaling before retinal injury decreases cell death and increases neuronal survival. a, b, Representative images of TUNEL-positive nuclei in vertical sections of retinas for eyes that were treated with vehicle before NMDA (a) or DAPT before NMDA (b). c, d, Histograms illustrate the mean (±SEM) number of dying cells in the INL in central, nasal, or temporal regions of the retina (c, d). Numbers of TUNEL+ cells were counted in retinas treated with 150 μg of NMDA (c) or 30 μg of NMDA (d) with and without DAPT pretreatment. e, f, Representative images of whole-mounted retinas that were treated with vehicle before colchicine (e) or DAPT before colchicine (f). Retinas were labeled with antibodies to Brn3a to identify ganglion cells. Histograms illustrate the mean (±SEM) number of ganglion cells per 0.55 mm2 in central, dorsal, nasal, or temporal regions of the retina (g, h). Datasets were obtained from 6 animals per treatment, per time point. Significance of difference (*p < 0.05, **p < 0.01) was determined by using a two-tailed, unpaired Student's t test. ONL, Outer nuclear layer; INL, inner nuclear layer. The scale bar (50 μm) in a applies to a and b, and that in f applies to e and f.
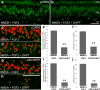
Figure 4.
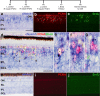
cNotch1 is upregulated by proliferating Müller glia in retinas treated with insulin and FGF2. Eyes were injected with three consecutive daily doses of insulin and FGF2 or saline (control) starting at P4, injected with BrdU at P7, and harvested at P8. a–c, Vertical sections of retina were hybridized with riboprobes to cNotch1 (a; blue) and subsequently labeled for PCNA (b; red) and BrdU (c; green). d is a merged image of a–c. e–g are 3.5-fold enlargements of corresponding boxed areas in d. h–j, Control retinas did not show an upregulation of cNotch1 (h), PCNA (i), or BrdU (j). White arrows in b indicate PCNA-positive cells. Yellow arrows in c and d indicate BrdU-positive cells. The asterisks (*) in e–g indicate cells labeled for cNotch1, PCNA, and BrdU. ONL, Outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer. The scale bar (50 μm) in a applies to a–c and h–j, and the scale bar in d (50 μm) applies to d alone.
Figure 5.
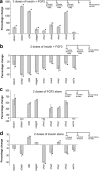
Notch and related genes are upregulated in retinas treated with insulin and FGF2. a–d, mRNA was harvested from retinas obtained from eyes treated with saline (control) or 3 consecutive daily doses of insulin and FGF2 (a), saline (control) or 2 consecutive daily doses of insulin and FGF2 (b), saline (control) or FGF2 alone (c), and saline (control) or insulin alone (d). Individual doses of insulin and FGF2 were 1000 and 250 ng, respectively. mRNA was reverse transcribed to generate cDNA and amplified using real-time PCR. Ct values were normalized to GAPDH, and the fold difference between control and treated samples was determined using the ΔCt method and represented as a percentage change (a–d). Significance of difference (*p < 0.03) was determined by using a two-tailed, unpaired Student's t test. RT, Reverse transcriptase.
Figure 6.
FGF2 induces the proliferation of Müller glia in injured retinas. Eyes were treated with 150 μg of NMDA at P4 followed by two consecutive daily doses of saline (control) or 250 ng of FGF2 (treated) at P5 and P6 and a dose of BrdU at P6, and harvested 24 h later at P7. a–c, e–g, Vertical sections of the P7 retina were labeled for Sox9 (a, e; magenta), BrdU (b, f; green), and PCNA (c, g; red). d is a merged image of a–c. h is a merged image of e–g. d and h are 3.5-fold enlargements of a–c and e–g, respectively. Arrows in e–h indicate Sox9-, BrdU-, and PCNA-triple-positive cells. The histograms in i represent the mean (±SEM) number of BrdU- and PCNA-positive cells per 15,000 μm2 of retinas. j–o, p–u, Vertical sections from control and treated retinas were labeled for 2M6 (red) and p38 MAPK (green; j–o) or pCREB (green; p–u). Significance of difference (**p < 0.001) was determined by using a two-tailed, unpaired Student's t test. Arrows indicate 2M6-positive Müller glia labeled for p38 MAPK (m–o) or pCREB (s–u). INL, Inner nuclear layer. The scale bar (50 μm) in g applies to a–c and e–g, and that in u applies to j–u.
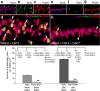
Figure 7.
Inhibition of Notch signaling reduces levels of pCREB and p38 MAPK in Müller glia. DAPT suppresses the accumulation of p38 MAPK and pCREB in Müller glia that results from NMDA+FGF2 treatment. Images were obtained using identical camera exposures and microscope illumination settings. Retinas were processed for immunolabeling after treatment with 150 μg of NMDA and two consecutive daily doses of 250 ng of FGF2 (control) or 250 ng of FGF2 + 865 ng of DAPT (treated). As described in the Materials and Methods, ImagePro 6.2 was used to obtain measurements of total area for pixel intensities >72 for pCREB and >65 for p38 MAPK (0 = black, 255 = saturated green), and the density sum. The small numbers and red areas in c, d, g, and h indicate the pixels designed by ImagePro 6.2 that met the threshold criteria. Means and SDs are displayed in histograms for total area (e, i) and density sum (f, j) for areas with pixel values above threshold. Significance of difference (**p < 0.001) was determined by using a two-tailed, unpaired Student's t test. ONL, Outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer. The scale bar (50 μm) in b applies to a and b, and that in h applies to c–h.
Figure 8.
Inhibition of Notch signaling blocks FGF2-induced proliferation of Müller glia in NMDA-damaged retinas. Eyes were injected with 150 μg of NMDA before or after two consecutive daily doses of 250 ng of FGF2 (control) or 250 ng of FGF2 and 865 ng of DAPT (treated), a dose of BrdU at P6 (or P7), and harvested at P7. a–c, e–g, Vertical sections of the P7 retina were labeled for Sox9 (a, e; magenta), BrdU (b, f; green), and PCNA (c, g; red). d is a merged image of a–c. h is a merged image of e–g. d and h are fourfold enlargements of a–c and e–g, respectively. Arrows in a–h indicate Sox9-, BrdU-, and PCNA-positive triple-labeled cells, and arrowheads indicate Sox9- and PCNA-double-positive cells. The histograms in i represent the mean (± SD) number of Sox9-, BrdU-, and PCNA-triple-positive cells per 15,000 μm2 of retina. Significance of difference (**p < 0.001) was determined by using a two-tailed, unpaired Student's t test. INL, Inner nuclear layer. The scale bar (50 μm) in g applies to a–c and e–g.
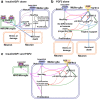
Figure 9.
Model for the influence of the Notch and MAPK pathways on retinal Müller glia. This figure draws from several studies to construct putative modes of action for insulin/IGF, FGF2, and neuronal damage on Müller glia in the chick retina (Fischer et al., 2002b, 2004b, 2009a,b). a, Effects of insulin/IGF1 alone: insulin/IGF1 signaling via the NIRG cells and/or microglia secondarily enhances effectors of MAPK signaling, i.e., p38 MAPK (IGF1-mediated) and cFos, Egr1, and pERK (insulin-mediated) in Müller glia in addition to increasing neuronal cell death (magenta arrows). It also modestly decreases Notch and related genes (magenta dashed arrows). Low levels of Notch signaling in Müller glia inhibits the neuron-supporting functions of Müller glia, exacerbating neuronal death (black arrows) during damage (orange arrows). In addition, low levels of Notch signaling enhance dedifferentiation and progenitor-like properties (black arrows), promoting Müller glial proliferation during damage. b, Effects of FGF2 alone: FGF2 has Notch-dependent and Notch-independent effects. (1) Notch-independent effects—FGF/MAPK signaling induces the accumulation of pERK1/2, p38 MAPK, pCREB, cfos, and Egr1 in Müller glia, which may stimulate the Müller glia to become neuroprotective and provide support to neurons in damaged retinas (blue arrows). pERK1/2 and Egr1 promote Müller glial dedifferentiation and proliferation in a Notch-independent manner (green arrows). (2) Notch-dependent effects—FGF/MAPK signaling induces upregulates expression of Notch and associated genes (pink dashed arrows). Low levels of Notch signaling in an undamaged or moderately damaged retina “prime” the glia to proliferate (black arrows). FGF2-mediated Müller glial proliferation requires some retinal damage and active Notch signaling, which promotes accumulation of p38 MAPK and pCREB (red arrows), which may make the glia more progenitor-like, inducing Müller glial proliferation during damage (orange arrow). c, Combined effects for three consecutive daily doses of insulin/IGF1 and FGF2: insulin/IGF1 signaling in the NIRG cells/microglia and FGF/MAPK signaling in the Müller glia together upregulate expression of Notch and its downstream effectors (pink arrows). Insulin/IGF1 signaling, FGF/MAPK signaling, and upregulated Notch signaling together induce the dedifferentiation and proliferation of Müller glia in the absence of damage.
Similar articles
- Turning Müller glia into neural progenitors in the retina.
Fischer AJ, Bongini R. Fischer AJ, et al. Mol Neurobiol. 2010 Dec;42(3):199-209. doi: 10.1007/s12035-010-8152-2. Epub 2010 Nov 20. Mol Neurobiol. 2010. PMID: 21088932 Review. - mTor signaling is required for the formation of proliferating Müller glia-derived progenitor cells in the chick retina.
Zelinka CP, Volkov L, Goodman ZA, Todd L, Palazzo I, Bishop WA, Fischer AJ. Zelinka CP, et al. Development. 2016 Jun 1;143(11):1859-73. doi: 10.1242/dev.133215. Epub 2016 Apr 11. Development. 2016. PMID: 27068108 Free PMC article. - Notch signaling regulates regeneration in the avian retina.
Hayes S, Nelson BR, Buckingham B, Reh TA. Hayes S, et al. Dev Biol. 2007 Dec 1;312(1):300-11. doi: 10.1016/j.ydbio.2007.09.046. Epub 2007 Sep 29. Dev Biol. 2007. PMID: 18028900 Free PMC article. - Mitogen-activated protein kinase-signaling stimulates Müller glia to proliferate in acutely damaged chicken retina.
Fischer AJ, Scott MA, Tuten W. Fischer AJ, et al. Glia. 2009 Jan 15;57(2):166-81. doi: 10.1002/glia.20743. Glia. 2009. PMID: 18709648 Free PMC article. - Potential of Müller Glia for Retina Neuroprotection.
Eastlake K, Luis J, Limb GA. Eastlake K, et al. Curr Eye Res. 2020 Mar;45(3):339-348. doi: 10.1080/02713683.2019.1648831. Epub 2019 Aug 5. Curr Eye Res. 2020. PMID: 31355675 Review.
Cited by
- Heterogeneity of glia in the retina and optic nerve of birds and mammals.
Fischer AJ, Zelinka C, Scott MA. Fischer AJ, et al. PLoS One. 2010 Jun 17;5(6):e10774. doi: 10.1371/journal.pone.0010774. PLoS One. 2010. PMID: 20567503 Free PMC article. - Fatty acid-binding proteins and fatty acid synthase influence glial reactivity and promote the formation of Müller glia-derived progenitor cells in the chick retina.
Campbell WA, Tangeman A, El-Hodiri HM, Hawthorn EC, Hathoot M, Blum S, Hoang T, Blackshaw S, Fischer AJ. Campbell WA, et al. Development. 2022 Mar 1;149(5):dev200127. doi: 10.1242/dev.200127. Epub 2022 Mar 4. Development. 2022. PMID: 35132991 Free PMC article. - Turning Müller glia into neural progenitors in the retina.
Fischer AJ, Bongini R. Fischer AJ, et al. Mol Neurobiol. 2010 Dec;42(3):199-209. doi: 10.1007/s12035-010-8152-2. Epub 2010 Nov 20. Mol Neurobiol. 2010. PMID: 21088932 Review. - Comparative Biology of Vertebrate Retinal Regeneration: Restoration of Vision through Cellular Reprogramming.
Todd L, Reh TA. Todd L, et al. Cold Spring Harb Perspect Biol. 2022 Jun 14;14(6):a040816. doi: 10.1101/cshperspect.a040816. Cold Spring Harb Perspect Biol. 2022. PMID: 34580118 Free PMC article. Review. - Cannabinoid signaling promotes the de-differentiation and proliferation of Müller glia-derived progenitor cells.
Campbell WA, Blum S, Reske A, Hoang T, Blackshaw S, Fischer AJ. Campbell WA, et al. Glia. 2021 Oct;69(10):2503-2521. doi: 10.1002/glia.24056. Epub 2021 Jul 7. Glia. 2021. PMID: 34231253 Free PMC article.
References
- Ahmad I, Zaqouras P, Artavanis-Tsakonas S. Involvement of Notch-1 in mammalian retinal neurogenesis: association of Notch-1 activity with both immature and terminally differentiated cells. Mech Dev. 1995;53:73–85. - PubMed
- Anezary L, Medina JI, Sánchez-Nogueiro J, López-Gallardo M, Prada C. Shape diversity among chick retina Muller cells and their postnatal differentiation. J Comp Neurol. 2001;438:32–49. - PubMed
- Austin CP, Feldman DE, Ida JA, Jr, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources