Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis - PubMed (original) (raw)
Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis
Matthew A Ciorba et al. J Immunol. 2010.
Abstract
The chronic inflammatory bowel diseases are characterized by aberrant innate and adaptive immune responses to commensal luminal bacteria. In both human inflammatory bowel disease and in experimental models of colitis, there is an increased expression of the enzyme IDO. IDO expression has the capacity to exert antimicrobial effects and dampen adaptive immune responses. In the murine trinitrobenzene sulfonic acid model of colitis, inhibition of this enzyme leads to worsened disease severity, suggesting that IDO acts as a natural break in limiting colitis. In this investigation, we show that induction of IDO-1 by a TLR-9 agonist, immunostimulatory (ISS) DNA, critically contributes to its colitis limiting capacities. ISS DNA induces intestinal expression of IDO-1 but not the recently described paralog enzyme IDO-2. This induction occurred in both epithelial cells and in subsets of CD11c(+) and CD11b(+) cells of the lamina propria, which also increase after ISS-oligodeoxynucleotide. Signaling required for intestinal IDO-1 induction involves IFN-dependent pathways, as IDO-1 was not induced in STAT-1 knockout mice. Using both the trinitrobenzene sulfonic acid and dextran sodium sulfate models of colitis, we show the importance of IDO-1s induction in limiting colitis severity. The clinical parameters and histological correlates of colitis in these models were improved by administration of the TLR-9 agonist; however, when the function of IDO is inhibited, the colitis limiting effects of ISS-oligodeoxynucleotide were abrogated. These findings support the possibility that targeted induction of IDO-1 is an approach deserving further investigation as a therapeutic strategy for diseases of intestinal inflammation.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest
Figures
Figure 1
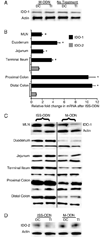
Intestinal tract IDO-1, but not IDO-2, is induced by ISS-ODN. 10 µg of either the ISS-ODN or the M-ODN was injected i.p. and the tissues were harvested at 72 hours for protein. Compared to control (untreated mice), the administration of M-ODN does not lead to induction of IDO-1 in either the colon or the small intestine (A). However, the injection of ISS-ODN leads to a significant induction of IDO-1 mRNA and protein throughout the intestinal tract and MLNs when compared to control mice injected with M-ODN (B,C). Analysis of the same samples did not show an increase in IDO-2 protein after ISS-ODN (D). All experiments consisted of at least 4 mice/group and were repeated at least twice. Two representative samples are shown in each western blot for figure 1C. RT-PCR data is shown as fold change relative to control ± SEM. * represents statistical significance of p≤0.05.
Figure 2
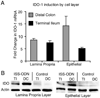
ISS-ODN administration leads to increased expression of IDO-1 in both lamina propria and epithelial cells layers. 10 µg of the ISS-ODN or the control M-ODN was injected i.p. into SJL/J mice and the tissues were harvested at 36 hours for RNA or 72 hours for protein. The tissue was processed to isolate the epithelial cell layer (via CMF-HBSS + EDTA) and the lamina propria cell layer (via Dispase/Collagenase digestion). Induction of IDO-1 mRNA by ISS-ODN is shown in each cell layer from both the colon and small intestine (A). An increase in IDO-1 protein is confirmed on this western blot for two representative samples from isolated cell layers from the colon and small intestine (B). This experiment was repeated twice with 2 mice in each group.
Figure 3
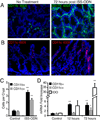
Cellular localization of IDO after ISS-ODN. Colon and small intestinal sections from control (M-ODN treated) mice shows faint staining for IDO-1 (red) (A,B; first image in each). Cytokeritin 18 (green) staining is used on colon sections to highlight structure. Dapi was used for nuclear staining. 72 hours after ISS-ODN administration IDO staining becomes markedly more positive in both epithelial cells (particularly at the base of the colonic crypts) as well as within the lamina propria and Peyer’s patches (A,B; images to right of arrow in each). Dual staining of frozen sections shows IDO-1 co-staining in some, but not all cells with CD11c (C) and CD11b (D), markers for dendritic cells and macrophages respectively. Staining for IDO-2 (red) was performed on the same control and ISS-ODN treated tissues. Faintly positive cells were identified in the lamina propria with no difference identified between the treated and untreated tissues (E).
Figure 4
CD11b and CD11c expressing cells increase after ISS-ODN administration. WT mice were administered one (12 hours prior) or two doses (12 and 72 hours prior) of ISS-ODN (20 µg). Immunoflorescence on frozen sections showed an increase in colon lamina propria CD11b and CD11c expressing cells (A,B). The number of positive staining cells in twenty crypts of three colonic cross sections from three different mice per group was counted. The average number of both cell types per crypt was increased (C). qRT-PCR showed a significant increased in only CD11b at 12 hours, but a significant increase in CD11b, CD11c and IDO-1 mRNA was apparent at 72 hours (D). n=4 mice/group repeated twice. * p<0.05, **p<0.01
Figure 5
Colon cytokine expression changes associated with ISS-ODN. Mice were administered one (12 hours prior) or two doses (12 and 72 hours prior) of ISS-ODN (20 µg) and tissues were harvested and analyzed by qRT-PCR for changes in cytokine expression. Cytokines known to induce IDO-1 including TNFα, IFNγ, and IFNα were significantly increased at both time points. IL-1B and IL-10 were also significantly induced at the 12, but not the 72 hour time point. n=4 mice/group repeated twice. *p<0.05
Figure 6
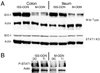
ISS-ODN induces IDO-1 in a STAT-1 dependent manner. Age matched WT C57Bl/6 mice and STAT-1 KO mice on a C57Bl/6 background were given 10 ug ISS-ODN or control M-ODN. IDO-1 expression increases in both the small intestine and colon of WT C57B/6 mice but not in the STAT1 KO mice (A). Two representative mice are shown for each condition. In the WT mice, activation of STAT-1 signaling by phosphorylation is shown on this western blot (B).
Figure 7
The clinical, histologic and morphologic benefits offered by ISS-ODN prior to TNBS colitis are abrogated by concurrent IDO inhibition. ISS-ODN was offered at 10 µg IP prior to TNBS or ethanol enema. The 1-mT slow release pellet was inserted concurrently with the TNBS enema. N=10 in each group. Relative weight changes from baseline weight are graphed as mean change ± SEM (A). TNBS enema was at 0.5 mg/mouse. Survival curve of mice with the same treatment groups is shown (B), where TNBS was administered at 0.7 mg/mouse. Distal colon sections were harvested 5 days after treatment and representative sections are shown at 100X stained with H&E (C).
Figure 8
IDO-1 induction is key to ISS-ODN’s protection against DSS colitis. 8 week old C57B/6 mice were offered 2.0% DSS in drinking water. On the day DSS was started, mice received 10 µg of ISS-ODN and/or 1-mT as listed. N=5 mice/group and the experiment was repeated 3 times with averaged composite values being shown in the figure. On day 7 disease activity index scores were compiled and the colons were subsequently harvested to test myeloperoxidase activity and fixed for histology evaluation of the distal rectum and proximal descending colon. The MPO activity (A), DAI (B), and colitis severity (C,D) were all significantly lower in the mice treated with ISS-ODN. No difference was found if 1-mT was administered alone. However, the addition of 1-mT eliminated the protective effect of each parameter which was improved by ISS-ODN. Representative histology sections (40X paired images) are shown in the bottom panel with the arrow marking the anorectal junction. Regenerative crypts (arrowhead (C)) with proliferating epithelial cells by Ki-67 staining (E) were more prevalent in the distal rectum of ISS-ODN treated mice. Similarly, this effect was lost with 1-mT co-administration. (p-values for histology: _a_=0.039 and _b_=0.001 vs DSS controls. _c_=0.003 and _d_=0.004 vs DSS + ISS-ODN treated groups)
Similar articles
- Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice.
Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Gurtner GJ, et al. Gastroenterology. 2003 Dec;125(6):1762-73. doi: 10.1053/j.gastro.2003.08.031. Gastroenterology. 2003. PMID: 14724829 - Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis.
Rachmilewitz D, Karmeli F, Takabayashi K, Hayashi T, Leider-Trejo L, Lee J, Leoni LM, Raz E. Rachmilewitz D, et al. Gastroenterology. 2002 May;122(5):1428-41. doi: 10.1053/gast.2002.32994. Gastroenterology. 2002. PMID: 11984528 - Glycogen synthase kinase 3-β: a master regulator of toll-like receptor-mediated chronic intestinal inflammation.
Hofmann C, Dunger N, Schölmerich J, Falk W, Obermeier F. Hofmann C, et al. Inflamm Bowel Dis. 2010 Nov;16(11):1850-8. doi: 10.1002/ibd.21294. Inflamm Bowel Dis. 2010. PMID: 20848477 - Inflamed intestinal mucosa features a specific epithelial expression pattern of indoleamine 2,3-dioxygenase.
Ferdinande L, Demetter P, Perez-Novo C, Waeytens A, Taildeman J, Rottiers I, Rottiers P, De Vos M, Cuvelier CA. Ferdinande L, et al. Int J Immunopathol Pharmacol. 2008 Apr-Jun;21(2):289-95. doi: 10.1177/039463200802100205. Int J Immunopathol Pharmacol. 2008. PMID: 18547472 - Immunoregulatory molecules are master regulators of inflammation during the immune response.
de la Fuente H, Cibrián D, Sánchez-Madrid F. de la Fuente H, et al. FEBS Lett. 2012 Aug 31;586(18):2897-2905. doi: 10.1016/j.febslet.2012.07.032. Epub 2012 Jul 20. FEBS Lett. 2012. PMID: 22819828 Free PMC article. Review.
Cited by
- IDO1 and IDO2 non-synonymous gene variants: correlation with crohn's disease risk and clinical phenotype.
Lee A, Kanuri N, Zhang Y, Sayuk GS, Li E, Ciorba MA. Lee A, et al. PLoS One. 2014 Dec 26;9(12):e115848. doi: 10.1371/journal.pone.0115848. eCollection 2014. PLoS One. 2014. PMID: 25541686 Free PMC article. - Dual activation of Toll-like receptors 7 and 9 impairs the efficacy of antitumor vaccines in murine models of metastatic breast cancer.
Moreno Ayala MA, Gottardo MF, Gori MS, Nicola Candia AJ, Caruso C, De Laurentiis A, Imsen M, Klein S, Bal de Kier Joffé E, Salamone G, Castro MG, Seilicovich A, Candolfi M. Moreno Ayala MA, et al. J Cancer Res Clin Oncol. 2017 Sep;143(9):1713-1732. doi: 10.1007/s00432-017-2421-7. Epub 2017 Apr 21. J Cancer Res Clin Oncol. 2017. PMID: 28432455 - Modeling colitis-associated cancer with azoxymethane (AOM) and dextran sulfate sodium (DSS).
Thaker AI, Shaker A, Rao MS, Ciorba MA. Thaker AI, et al. J Vis Exp. 2012 Sep 11;(67):4100. doi: 10.3791/4100. J Vis Exp. 2012. PMID: 22990604 Free PMC article. - The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists.
Thomas S, Izard J, Walsh E, Batich K, Chongsathidkiet P, Clarke G, Sela DA, Muller AJ, Mullin JM, Albert K, Gilligan JP, DiGuilio K, Dilbarova R, Alexander W, Prendergast GC. Thomas S, et al. Cancer Res. 2017 Apr 15;77(8):1783-1812. doi: 10.1158/0008-5472.CAN-16-2929. Epub 2017 Mar 14. Cancer Res. 2017. PMID: 28292977 Free PMC article. Review. - IDO1 scavenges reactive oxygen species in myeloid-derived suppressor cells to prevent graft-versus-host disease.
Ju JM, Nam G, Lee YK, Jung M, Chang H, Kim W, Shon WJ, Lim JY, Kim JY, Chang J, Min CK, Lee DS, Choi K, Shin DM, Choi EY. Ju JM, et al. Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2011170118. doi: 10.1073/pnas.2011170118. Proc Natl Acad Sci U S A. 2021. PMID: 33649207 Free PMC article.
References
- MacKenzie CR, Heseler K, Muller A, Daubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab. 2007;8:237–244. - PubMed
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. - PubMed
- Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5605. - PubMed
- Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. - PubMed
- Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, McQuillan JA, Stocker R, Jermiin LS, Hunt NH. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- L30 RR030244/RR/NCRR NIH HHS/United States
- DK075713/DK/NIDDK NIH HHS/United States
- P30 DK52574/DK/NIDDK NIH HHS/United States
- DK064798/DK/NIDDK NIH HHS/United States
- L30 RR030244-01/RR/NCRR NIH HHS/United States
- R01 DK064798-07/DK/NIDDK NIH HHS/United States
- R01 DK075713/DK/NIDDK NIH HHS/United States
- R01 DK064798/DK/NIDDK NIH HHS/United States
- R01 CA109542/CA/NCI NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous