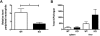STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction - PubMed (original) (raw)
STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction
Hoainam Nguyen-Jackson et al. Blood. 2010.
Abstract
Neutrophil mobilization, the release of neutrophils from the bone marrow reserve into circulating blood, is important to increase peripheral neutrophil amounts during bacterial infections. Granulocyte colony-stimulating factor (G-CSF) and chemokines, such as macrophage-inflammatory protein-2 (MIP-2; CXCL2), can induce neutrophil mobilization, but the mechanism(s) they use remain unclear. Signal transducers and activator of transcription 3 (STAT3) is the principal intracellular signaling molecule activated upon G-CSF ligation of its receptor. Using a murine model with conditional STAT3 deletion in bone marrow, we demonstrated previously that STAT3 regulates acute G-CSF-responsive neutrophil mobilization and MIP-2-dependent neutrophil chemotaxis. In this study, we show STAT3 is also necessary for MIP-2-elicited neutrophil mobilization. STAT3 appears to function by controlling extracellular signal-regulated kinase (ERK) activation, which is important for MIP-2-mediated chemotaxis. In addition, we demonstrate that G-CSF stimulates the expression of the MIP-2 receptor via STAT3-dependent transcriptional activation of Il8rb. G-CSF treatment also induces STAT3-dependent changes in bone marrow chemokine expression levels which may further affect neutrophil retention and release. Taken together, our study demonstrates that STAT3 regulates multiple aspects of chemokine and chemokine receptor expression and function within the bone marrow, indicating a central role in the neutrophil mobilization response.
Figures
Figure 1
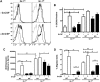
STAT3-dependent regulation of neutrophil migration and CXCR2-mediated Raf/MEK/ERK signaling. (A) Peripheral blood neutrophil numbers were determined for WT (□) and STAT3-deficient (knockout [KO], ■) mice treated with MIP-2 (50 μg/kg) or BSA carrier for 30 minutes. Neutrophil amounts in MIP-2–treated mice were normalized to BSA-treated controls of the appropriate genotype and relative levels are shown (n = at least 4 for WT and KO). (B) Absolute neutrophil numbers in total spleen, blood, and bone marrow of BSA- or MIP-2–treated mice are shown (n = at least 3 for WT and KO for each condition). (C) CXCR2 expression within the Gr-1+ bone marrow population of BSA- and MIP-2–treated WT (black line) and STAT3-deficient (KO, gray line) mice is shown, relative to isotype controls (dashed black line). Results are representative of 3 independent experiments. (D) Activation of c-Raf, MEK1/2, and ERK1/2 in MIP-2–treated (100 ng/mL) or unstimulated Gr-1+ cells was analyzed by immunoblotting and quantified by densitometry. Results are representative of at least 3 independent experiments. (E) MIP-2–responsive chemotaxis of bone marrow neutrophils from WT mice was examined in the absence of or after, 30 minutes pretreatment with the MEK1/2 inhibitor U0126 (10μM or 50μM as indicated, n = 5 for each condition). Average values from 3 independent experiments are shown. (F) WT bone marrow neutrophils were stimulated with MIP-2 (100 ng/mL for 5 minutes) in the presence or absence of U0126 (10μM or 50μM as indicated). ERK1/2 activation was assessed by immunoblotting with whole-cell lysates; results are representative of 3 independent experiments. Error bars represent SEM; *P < .05, **P < .01, ***P < .001.
Figure 2
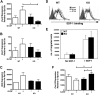
Role for STAT3 in G-CSF–inducible CXCR2 expression. (A) WT and STAT3-deficient (KO) mice were treated with G-CSF (250 μg/kg; +G-CSF, bottom) or left untreated (−G-CSF, top) and bone marrow samples were isolated after 24 hours. CXCR2 expression in the Gr-1+ population is shown: WT (black line), KO (gray line). Isotype controls are included (dashed gray line). Data shown are from a representative experiment (n = at least 3 for each group). (B) The average percentage of CXCR2-positive cells within the bone marrow Gr-1hi and Gr-1lo subsets from untreated (−G-CSF) or G-CSF–treated (+G-CSF) WT and STAT3-deficient (KO) mice is shown (n = at least 3 for each group). (C) WT and STAT3-deficient (KO) mice were untreated or treated with G-CSF as indicated in panel A. Relative Il8rb mRNA levels were determined in purified Gr-1lo and Gr-1hi subsets, by comparison with the housekeeping gene Rpl13a. Shown are mean relative expression levels (n = at least 4). (D) WT and STAT3-deficient (KO) mice were untreated or treated with G-CSF as indicated in panel A. MIP-2–responsive chemotactic activity of purified immature Gr-1lo and mature Gr-1hi neutrophils was determined by Transwell assays. The average percentage of migrated cells/total cells is shown for each condition (n = 5 for each group). Error bars represent SEM; *P < .05, **P < .01, ***P < .001.
Figure 3
Regulation of Il8rb transcription by G-CSF–responsive STAT3 signaling. (A) A schematic of the Il8rb gene showing the location of the STATx site (top) and sequence of the mutant STAT element (bottom). (B) 32D.G-CSFR cells were electroporated with WT (WT STAT) or mutant pGL3-Il8rb (mutant STAT) and pTK-Renilla reporter plasmids, treated with or without G-CSF for 6 hours, and assayed for luciferase activity. The ratio of firefly:renilla relative light units (RLU) in G-CSF–treated cells relative to unstimulated cells (−G-CSF) was averaged from 3 independent experiments. (C) EMSAs were performed with nuclear extracts from 32D.G-CSFR cells, stimulated with or without G-CSF for 30 minutes by the use of radiolabeled oligonucleotide probes corresponding to the Il8rb promoter region containing the STATx site (WT Il8rb) or an oligonucleotide containing a mutation in the STATx site of the Il8rb promoter (mutant Il8rb), as indicated. An excess of unlabeled oligonucleotide, corresponding to the WT Il8rb probe (Il8rb), an oligonucleotide encompassing the STAT3 binding site in Socs3 (Socs3), or an oligonucleotide containing a mutation in the STAT3 site from Socs3 (m_Socs3_) was used as competitor, as indicated. Results are representative of 3 independent experiments. (D) EMSAs were performed as in panel C with the WT Il8rb probe in the absence or presence of anti-STAT3 antibody. Results are representative of 3 independent experiments. (E) ChIPs were performed on 32D.G-CSFR cells ± G-CSF treatment for 30 minutes with anti-STAT3 or Ig control antibody, as indicated. Purified DNA samples were subjected to PCR to detect Il8rb (top) or Socs3 (bottom) promoter sequences. Data are representative of 3 independent experiments. Error bars represent SEM.
Figure 4
G-CSF treatment results in STAT3-dependent alterations in the chemokine profile of the bone marrow microenvironment. WT or STAT3-deficient (KO) mice were injected with G-CSF or left untreated. Total bone marrow cells were isolated after 24 hours, and purified RNA samples were analyzed by real-time PCR for (A) MIP-2 (Cxcl2), (B) KC (Cxcl1), or (C) CXCR2 (Il8rb) expression. Gene expression was determined relative to Rpl13a (n = at least 4). (D) SDF-1α binding assays were performed with purified bone marrow neutrophils from WT or STAT-deficient (KO) mice either by the use of freshly isolated neutrophils (0 hrs, open black histograms) or neutrophils cultured ex vivo in G-CSF for 6 hours (6 hours, open gray histograms). Staining with human-Fc (solid gray histograms) was used as a control. Results are representative of 3 independent experiments. (E) Neutrophils isolated from the bone marrow of WT (□) or KO (■) mice were tested for SDF-1–dependent chemotaxis (n = 4 for each genotype). Average values from 3 independent experiments are shown. (F) WT or STAT-deficient (KO) mice were treated with G-CSF or left untreated. SDF-1 (Cxcl12) mRNA expression was determined in total bone marrow 24 hours after treatment by the use of real-time PCR as in panels A to C (n = at least 4). Error bars represent SEM; *P < .05, **P < .01.
Figure 5
Impaired mobilization of STAT3-deficient neutrophils during Listeria monocytogenes infection. WT or STAT3-deficient (KO) mice were infected with L monocytogenes by intravenous injection, as described in “Methods.” (A) At 12 hours after infection, circulating neutrophil numbers in peripheral blood were determined by automated counting. Data shown are average fold change in peripheral neutrophil numbers in infected versus uninfected animals (n = 5 for WT, n = 4 for KO). (B) Spleens and livers were isolated 12 hours after infection with L monocytogenes, homogenized, and cultured. CFUs were enumerated 24 hours after culture. Shown are mean CFU/organ (n = 6 for WT, n = 4 for KO). Error bars represent SEM *P < .05.
Figure 6
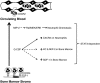
A model for STAT3 function in neutrophil mobilization. Neutrophils are retained in the bone marrow in part through their expression of CXCR4, which interacts with SDF-1 expressed by stromal cells. G-CSF treatment directly or indirectly down-regulates CXCR4 and SDF-1; repression of SDF-1 requires STAT3 (dashed line). G-CSF treatment also induces the neutrophil chemoattractants KC and MIP-2 in the bone marrow (dashed line), as well as concurrent up-regulation of their shared receptor CXCR2 on the surface of neutrophils (solid line); induction of KC, MIP-2 and CXCR2 each require STAT3. STAT3 also controls the amplitude of MIP-2-induced Raf/MEK/ERK signaling (dashed line), which is crucial for neutrophil chemotaxis. Solid line denotes direct molecular regulation by STAT3; dashed lines indicate STAT3 regulation may be direct or indirect.
Similar articles
- G-CSF-activated STAT3 enhances production of the chemokine MIP-2 in bone marrow neutrophils.
Nguyen-Jackson HT, Li HS, Zhang H, Ohashi E, Watowich SS. Nguyen-Jackson HT, et al. J Leukoc Biol. 2012 Dec;92(6):1215-25. doi: 10.1189/jlb.0312126. Epub 2012 Sep 27. J Leukoc Biol. 2012. PMID: 23024284 Free PMC article. - G-CSF maintains controlled neutrophil mobilization during acute inflammation by negatively regulating CXCR2 signaling.
Bajrami B, Zhu H, Kwak HJ, Mondal S, Hou Q, Geng G, Karatepe K, Zhang YC, Nombela-Arrieta C, Park SY, Loison F, Sakai J, Xu Y, Silberstein LE, Luo HR. Bajrami B, et al. J Exp Med. 2016 Sep 19;213(10):1999-2018. doi: 10.1084/jem.20160393. Epub 2016 Aug 22. J Exp Med. 2016. PMID: 27551153 Free PMC article. - The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation.
Wengner AM, Pitchford SC, Furze RC, Rankin SM. Wengner AM, et al. Blood. 2008 Jan 1;111(1):42-9. doi: 10.1182/blood-2007-07-099648. Epub 2007 Oct 10. Blood. 2008. PMID: 17928531 Free PMC article. - Neutrophil mobilization and clearance in the bone marrow.
Furze RC, Rankin SM. Furze RC, et al. Immunology. 2008 Nov;125(3):281-8. doi: 10.1111/j.1365-2567.2008.02950.x. Immunology. 2008. PMID: 19128361 Free PMC article. Review. - Peripheral blood stem cell mobilization: the CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties.
Pelus LM, Fukuda S. Pelus LM, et al. Exp Hematol. 2006 Aug;34(8):1010-20. doi: 10.1016/j.exphem.2006.04.004. Exp Hematol. 2006. PMID: 16863907 Review.
Cited by
- G-CSF enhances resolution of Staphylococcus aureus wound infection in an age-dependent manner.
Brubaker AL, Kovacs EJ. Brubaker AL, et al. Shock. 2013 Oct;40(4):327-33. doi: 10.1097/SHK.0b013e3182a43651. Shock. 2013. PMID: 23856924 Free PMC article. - Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing.
Sawaya AP, Stone RC, Brooks SR, Pastar I, Jozic I, Hasneen K, O'Neill K, Mehdizadeh S, Head CR, Strbo N, Morasso MI, Tomic-Canic M. Sawaya AP, et al. Nat Commun. 2020 Sep 16;11(1):4678. doi: 10.1038/s41467-020-18276-0. Nat Commun. 2020. PMID: 32938916 Free PMC article. - CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway.
Hou SM, Chen PC, Lin CM, Fang ML, Chi MC, Liu JF. Hou SM, et al. Arthritis Res Ther. 2020 Oct 21;22(1):251. doi: 10.1186/s13075-020-02331-8. Arthritis Res Ther. 2020. PMID: 33087182 Free PMC article. - Human hyper-IgE syndrome: singular or plural?
Zhang Q, Boisson B, Béziat V, Puel A, Casanova JL. Zhang Q, et al. Mamm Genome. 2018 Aug;29(7-8):603-617. doi: 10.1007/s00335-018-9767-2. Epub 2018 Aug 9. Mamm Genome. 2018. PMID: 30094507 Free PMC article. Review. - PMF-GRN: a variational inference approach to single-cell gene regulatory network inference using probabilistic matrix factorization.
Skok Gibbs C, Mahmood O, Bonneau R, Cho K. Skok Gibbs C, et al. Genome Biol. 2024 Apr 8;25(1):88. doi: 10.1186/s13059-024-03226-6. Genome Biol. 2024. PMID: 38589899 Free PMC article.
References
- Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest. 2000;80(5):617–653. - PubMed
- Newburger PE. Disorders of neutrophil number and function. Hematology Am Soc Hematol Educ Program. 2006:104–110. - PubMed
- Tian SS, Tapley P, Sincich C, Stein RB, Rosen J, Lamb P. Multiple signaling pathways induced by granulocyte colony-stimulating factor involving activation of JAKs, STAT5, and/or STAT3 are required for regulation of three distinct classes of immediate early genes. Blood. 1996;88(12):4435–4444. - PubMed
- Tian SS, Lamb P, Seidel HM, Stein RB, Rosen J. Rapid activation of the STAT3 transcription factor by granulocyte colony-stimulating factor. Blood. 1994;84(6):1760–1764. - PubMed
- Lee CK, Raz R, Gimeno R, et al. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17(1):63–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- T32 CA009598/CA/NCI NIH HHS/United States
- T32-CA-09598-16/CA/NCI NIH HHS/United States
- AI073587/AI/NIAID NIH HHS/United States
- R21 AI073587/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous