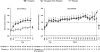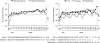Oral tolvaptan is safe and effective in chronic hyponatremia - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.1681/ASN.2009080857. Epub 2010 Feb 25.
Collaborators, Affiliations
- PMID: 20185637
- PMCID: PMC2844305
- DOI: 10.1681/ASN.2009080857
Randomized Controlled Trial
Oral tolvaptan is safe and effective in chronic hyponatremia
Tomas Berl et al. J Am Soc Nephrol. 2010 Apr.
Erratum in
- J Am Soc Nephrol. 2010 Aug;21(8):1407
Abstract
Vasopressin antagonists increase the serum sodium concentration in patients who have euvolemia and hypervolemia with hyponatremia in the short term (</=30 days), but their safety and efficacy with longer term administration is unknown. SALTWATER was a multicenter, open-label extension of the Study of Ascending Levels of Tolvaptan in Hyponatremia (SALT-1 and SALT-2). In total, 111 patients with hyponatremia received oral tolvaptan for a mean follow-up of 701 days, providing 77,369 patient-days of exposure. All patients had hyponatremia at randomization in SALT-1 and SALT-2, and 85% continued to have hyponatremia at entry in SALTWATER. The most common adverse effects attributed to tolvaptan were pollakiuria, thirst, fatigue, dry mouth, polydipsia, and polyuria. Six drug-related adverse effects led to study discontinuation. The increase in serum sodium exceeded the desired 1 mmol/L per h at initiation in five patients. Hypernatremia (>145 mmol/L) led to discontinuation in one patient. Mean serum sodium increased from 130.8 mmol/L at baseline to >135 mmol/L throughout the observation period (P < 0.001 versus baseline at most points). Responses were comparable between patients with euvolemia and those with heart failure but more modest in patients with cirrhosis. In conclusion, prolonged administration of tolvaptan maintains an increased serum sodium with an acceptable margin of safety.
Figures
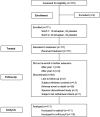
Figure 1.
SALTWATER flow diagram shows progression of study. aReceived at least one dose of tolvaptan; breceived at least one dose of tolvaptan and had a serum sodium assessment at baseline and at least one postbaseline time point.
Figure 2.
Serum sodium levels obtained in the course of the SALT-1, SALT-2 and SALTWATER trials. Rate of correction of serum sodium levels and the plateaus reached afterwards were similar in both studies. The left-hand figure includes only patients in the SALT-1 and SALT-2 who continued into SALTWATER. Error bars are ± SE. BSL, baseline; FU, follow-up visit 7 days after early withdrawal or trial completion. a_P_ < 0.001, tolvaptan versus placebo; b_P_ ≤ 0.0011, tolvaptan and tolvaptan (before placebo) versus baseline; c_P_ < 0.001, tolvaptan and tolvaptan (before placebo) versus baseline; d_P_ = 0.021, tolvaptan versus baseline and P < 0.001 tolvaptan (before placebo) versus baseline; e_P_ < 0.001, tolvaptan (before placebo) versus baseline; f_P_ = 0.005, tolvaptan (before placebo) versus baseline; g_P_ = 0.016 tolvaptan (before placebo) versus baseline.
Figure 3.
Serum sodium measured according to severity of hyponatremia (left) and underlying cause (right). The correction rate had similar kinetics for all groups, except a steeper initial response for the marked hyponatremia subgroup (left). Comparisons versus baseline were statistically significant (P < 0.05) for patients with mild hyponatremia at all time points but week 214 and the follow-up visit; for patients with marked hyponatremia at all visits but weeks 202 and 214; for patients with CHF at all time points but weeks 190, 202, and 214 and the follow-up visit; for patients with cirrhosis at 8 hours, day 31, weeks 10, 18, and 50, and the follow-up visit; and for patients with SIADH/other at all visits but week 214 and the follow-up visit.
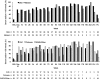
Figure 4.
Percentage of patients who normalized their serum sodium according to severity of hyponatremia (top) and etiology (bottom). Approximately half of patients exhibited normal serum sodium levels by week 4. Correction rates seemed to be generally similar among patients with CHF and SIADH/other but may have been somewhat lower among patients with cirrhosis.
Comment in
- Treatment of chronic hyponatremia: now we know how, but do we know when or if?
Greenberg A, Lehrich RW. Greenberg A, et al. J Am Soc Nephrol. 2010 Apr;21(4):552-5. doi: 10.1681/ASN.2010020157. Epub 2010 Feb 25. J Am Soc Nephrol. 2010. PMID: 20185636 No abstract available. - Hyponatremia: Prolonged use of the vasopressin antagonist tolvaptan is a safe and effective treatment for hyponatremia.
Ireland R. Ireland R. Nat Rev Nephrol. 2010 Jun;6(6):315. doi: 10.1038/nrneph.2010.61. Nat Rev Nephrol. 2010. PMID: 20508668 No abstract available.
Similar articles
- Tolvaptan use in cancer patients with hyponatremia due to the syndrome of inappropriate antidiuretic hormone: a post hoc analysis of the SALT-1 and SALT-2 trials.
Gralla RJ, Ahmad F, Blais JD, Chiodo J 3rd, Zhou W, Glaser LA, Czerwiec FS. Gralla RJ, et al. Cancer Med. 2017 Apr;6(4):723-729. doi: 10.1002/cam4.805. Epub 2017 Mar 2. Cancer Med. 2017. PMID: 28251822 Free PMC article. Clinical Trial. - Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis.
Cárdenas A, Ginès P, Marotta P, Czerwiec F, Oyuang J, Guevara M, Afdhal NH. Cárdenas A, et al. J Hepatol. 2012 Mar;56(3):571-8. doi: 10.1016/j.jhep.2011.08.020. Epub 2011 Oct 23. J Hepatol. 2012. PMID: 22027579 Clinical Trial. - Vaptans: a potential new approach for treating chronic hyponatremia in psychotic patients.
Josiassen RC, Curtis JL, Shaughnessy RA, Filmyer DM, Geboy AG, Skuban N, Ouyang J, Czerwiec F. Josiassen RC, et al. Clin Schizophr Relat Psychoses. 2012 Apr;6(1):21-6. doi: 10.3371/CSRP.6.1.3. Clin Schizophr Relat Psychoses. 2012. PMID: 22453866 Clinical Trial. - Tolvaptan.
Plosker GL. Plosker GL. Drugs. 2010 Mar 5;70(4):443-54. doi: 10.2165/11204630-000000000-00000. Drugs. 2010. PMID: 20205486 Review.
Cited by
- The Vasopressin Receptor Antagonist Tolvaptan Counteracts Tumor Growth in a Murine Xenograft Model of Small Cell Lung Cancer.
Naldi L, Fibbi B, Polvani S, Cirillo C, Pasella F, Bartolini F, Romano F, Fanelli A, Peri A, Marroncini G. Naldi L, et al. Int J Mol Sci. 2024 Aug 1;25(15):8402. doi: 10.3390/ijms25158402. Int J Mol Sci. 2024. PMID: 39125971 Free PMC article. - [Consensus recommendations on the diagnosis and treatment of hyponatremia from the Austrian Society for Nephrology 2024].
Schwarz C, Lindner G, Windpessl M, Knechtelsdorfer M, Saemann MD. Schwarz C, et al. Wien Klin Wochenschr. 2024 Feb;136(Suppl 1):1-33. doi: 10.1007/s00508-024-02325-5. Epub 2024 Feb 29. Wien Klin Wochenschr. 2024. PMID: 38421476 Free PMC article. German. - Hypertonic Saline Administration via Intraosseous Access During Symptomatic Hyponatremia.
Juarez A, Barr M, Golden T. Juarez A, et al. Cureus. 2023 Jul 11;15(7):e41731. doi: 10.7759/cureus.41731. eCollection 2023 Jul. Cureus. 2023. PMID: 37575736 Free PMC article. - Long-term low-dose tolvaptan efficacy and safety in SIADH.
Bondanelli M, Aliberti L, Gagliardi I, Ambrosio MR, Zatelli MC. Bondanelli M, et al. Endocrine. 2023 Nov;82(2):390-398. doi: 10.1007/s12020-023-03457-w. Epub 2023 Jul 28. Endocrine. 2023. PMID: 37507553 Free PMC article. - Tolvaptan for the treatment of the syndrome of inappropriate antidiuresis (SIAD).
Tzoulis P, Kaltsas G, Baldeweg SE, Bouloux PM, Grossman AB. Tzoulis P, et al. Ther Adv Endocrinol Metab. 2023 May 16;14:20420188231173327. doi: 10.1177/20420188231173327. eCollection 2023. Ther Adv Endocrinol Metab. 2023. PMID: 37214762 Free PMC article. Review.
References
- Greenberg A, Verbalis JG: Vasopressin receptor antagonists. Kidney Int 69: 2124– 2130, 2006 - PubMed
- Annane D, Decaux G, Smith N: Efficacy and safety of oral conivaptan, a vasopressin-receptor antagonist, evaluated in a randomized, controlled trial in patients with euvolemic or hypervolemic hyponatremia. Am J Med Sci 337: 28– 36, 2009 - PubMed
- Decaux G: Long-term treatment of patients with inappropriate secretion of antidiuretic hormone by the vasopressin receptor antagonist conivaptan, urea, or furosemide. Am J Med 110: 582– 584, 2001 - PubMed
- Ghali JK, Koren MJ, Taylor JR, Brooks-Asplund E, Fan K, Long WA, Smith N: Efficacy and safety of oral conivaptan: A V1A/V2 vasopressin receptor antagonist, assessed in a randomized, placebo-controlled trial in patients with euvolemic or hypervolemic hyponatremia. J Clin Endocrinol Metab 91: 2145– 2152, 2006 - PubMed
- Gines P, Wong F, Watson H, Milutinovic S, del Arbol LR, Olteanu D: Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: A randomized trial. Hepatology 48: 204– 213, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources