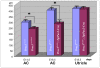Canal cristae growth and fiber extension to the outer hair cells of the mouse ear require Prox1 activity - PubMed (original) (raw)
Canal cristae growth and fiber extension to the outer hair cells of the mouse ear require Prox1 activity
Bernd Fritzsch et al. PLoS One. 2010.
Abstract
Background: The homeobox gene Prox1 is required for lens, retina, pancreas, liver, and lymphatic vasculature development and is expressed in inner ear supporting cells and neurons.
Methodology/principal findings: We have investigated the role of Prox1 in the developing mouse ear taking advantage of available standard and conditional Prox1 mutant mouse strains using Tg(Pax2-Cre) and Tg(Nes-Cre). A severe reduction in the size of the canal cristae but not of other vestibular organs or the cochlea was identified in the E18.5 Prox1(Flox/Flox); Tg(Pax2-Cre) mutant ear. In these mutant embryos, hair cell differentiated; however, their distribution pattern was slightly disorganized in the cochlea where the growth of type II nerve fibers to outer hair cells along Prox1 expressing supporting cells was severely disrupted. In the case of Nestin-Cre, we found that newborn Prox1(Flox/Flox); Tg(Nestin-Cre) exhibit only a disorganized innervation of outer hair cells despite apparently normal cellular differentiation of the organ of Corti, suggesting a cell-autonomous function of Prox1 in neurons.
Conclusions/significance: These results identify a dual role of Prox1 during inner ear development; growth of the canal cristae and fiber guidance of Type II fibers along supporting cells in the cochlea.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. The early onset of Prox1 expression is revealed by β-galactosidase expression and in situ hybridization.
Whole mount β-galactosidase histochemcial reaction using X-Gal was performed in Prox1 heterozygous and nullizygous embryos. A. Starting at E11.0, a progressive upregulation of Prox1 is seen in the anterior (AC) and posterior (PC) canal cristae. B. By E13.5, expression is also detected in the horizontal canal crista (HC), the striolar region of the utricle (U), the canals and the endolymphatic duct (ED); expression in the saccule is barely detected (S). In the cochlea, upregulation of β-galactosidase expression is detected in the apex and decreases toward the base. Arrows indicate expression in anterior and posterior canal with their expression. C. Expression of β-galactosidase is identical in heterozygous and nullizygous mice with the exception that the signal is stronger in nullizygous mice. Faint β-galactosidase expression is also detected in the delaminating spiral ganglion neurons (SPG; C and insert in B,C). D. In situ hybridization shows at E14.5 expression in the canal cristae and the cochlea, but indicates a more prominent upregulation in the base at this stage. Only spiral ganglion sensory neurons are faintly positive for Prox1 in situ (SPG in D). E,F At postnatal stages, Prox1 expression remains in the canal cristae as revealed by in situ hybridization for Prox1 mRNA or X-Gal reaction, but does not show the extensive expression in the non-sensory parts of the canals as in earlier stages (insert in F). Bar, 100 µm.
Figure 2. Effects of Prox1 loss-of function in the vestibular epithelia.
A. X-gal staining of E14.5 Prox1 heterozygous embryos reveals β-galactosidase activity in the anterior (AC) and horizontal (HC) parts of the canal cristae. B. Although morphologically normal, a reduction in the size of the crista epithelia is detected of _Prox1_-null littermates (white bar in the AC); gravistatic sensors such as utricle (U) show only transient Prox1 expression and no apparent reduction in size. C, E. Hair cells are revealed using antibodies against Myo VII in a normal E18.5 Prox1flox/flox conditional embryo. Note absence of imunoreactivity in the cruciate eminence (CE) of the anterior canal crista. E′. As shown by 2-photon activation, at this later stage, Prox1 expression is high in supporting cells, but is also found in hair cells of the canal cristae as well as outside the sensory epithelium. Dotted line in B indicate the plane of sections through the horizontal canal crista, white arrows align lateral walls of the whole mount with the section. E,F. Despite the overlap of some Prox1 expression with hair cells in the canal cristae there is no morphologically obvious defects in hair cell differentiation other than reduced intensity of Myo VII staining are observed in Prox1 flox/flox; Tg(Pax2-Cre) as compared to Prox1flox/flox littermates. However the reduction in size of the anterior canal crista (AC) is becoming more obvious at this late stage (C–F). CE-Cruciate eminence. Bar, 100 µm.
Figure 3. Prox1 inactivation reduces the size of the anterior crista.
As measured at E14.5, the length of the anterior cristae (AC) of Prox1 mutant embryos is 20% reduced when compare vs. that of wild-type littermates. The size reduction is 30% when compared with the size of E18.5 Prox1 flox/flox; Tg(Pax2-Cre) mutant embryos. No significant changes in the length of the utricle were observed. Asterisks indicate a level of significance (p<0.05; t-test).
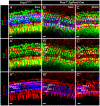
Figure 4. Prox1 expression in the cochlea is biphasic.
A. As shown by X-gal staining, at E13.5 Prox1 expression in the cochlea is higher in the apex and gradually faints toward the base; with limited expression in delaminating sensory neurons (SPG in A, A′). B,C. Expression is later on found throughout the organ of Corti. C. This elevated expression has not yet reached the undifferentiated apex (C, E,E′) which is confirmed by Prox1 in situ hybridization (C′) that also indicates Prox1 expression in the spiral ganglion (SPG; C′). D, D′. Whole mount analysis, including 2 photon activation of the β-galactosidase reaction product (D′) show that near the base the expression of Prox1 is nearly exclusive found in the five supporting cells of the lesser epithelial ridge (three rows of Deiter's cell, D1–3; two rows of pillar cells (IP, OP) with limited expression in some outer hair cells (arrow C′) and inner phalangeal cells (arrows in D, D′). E,E′. In the apex the expression of Prox1 is not restricted to just five rows of cells, reflecting the immature state of the apex with incomplete convergent extension and possible expanded expression of Prox1. F,G. Expression in supporting cells stays on in young adults and there is a faint expression in spiral ganglion cells (SPG; F). No labeling is found around inner hair cells (IHC) in postnatal animals (F–I). H, I. Prox1 expression was verified using in situ hybridization in newborn wildtype and Prox1 flox/flox; Tg(Pax2-Cre) conditional mutants. Note the prominent presence of the in situ signal in sensory neurons and the slight reduction of the overall signal in the organ of Corti in the conditional null mice (I) that is possibly related to the disorganization of supporting cells (see Fig. 5). The in situ hybridization will detect the full length and the conditionally truncated and non functional mRNA of Prox1. Immunocytochemistry on whole mounts (J,K) and sections (L) verifies the data obtained with X-Gal reaction and in situ hybiridization and reveals a prominent expression in supporting cells (J,J′, K,K′ L) and spiral ganglion neurons (SGN, J, J′). Myosin VII (Myo VII) stain hair cells (J″, K″, L) but not supporting cells. Bar, 100 µm.
Figure 5. Fiber growth to outer hair cells is defective in E18.5 Prox1 flox/flox; Tg(Pax2-Cre) mutant embryos.
A. Prox1 antibody staining reveals the normal expression of pattern of Prox1 in supporting cells (three rows of Deiter's cells, D1–3; two rows of pillar cells, IP, OP). There is also faint immunostaining in cells medial to the inner pillar cell (IP), probably in inner phalangeal cells. A′. Successful conditional inactivation of Prox1 is indicated by the barely detectable expression of Prox1 in Prox1 flox/flox; Tg(Pax2-Cre) mutant littermates at this stage. B, B′. Wildtype mice have four rows of hair cells (three rows of outer hair cells, OHC1–3, one row of inner hair cells, IHC). As seen by Myo VII staining, a partial fourth row and some misaligned outer hair cells (arrows) were occasionally detected in Prox1 flox/flox; Tg(Pax2-Cre) mutant embryos that is obvious in Hoechst stain with p75 labeling of pillar and Hensen cells (inserts). Otherwise, no other obvious changes in the distribution and maturation of Myo VII-expressing hair cells were detected. C, D. Normally and as seen by β-tubulin immunostaining, fibers grow out through the tunnel of Corti (TC) and turn to form three parallel outer spiral bundles (arrows) that run along Deiter's cells to reach the three rows of outer hair cells (OHC) in the base. C′, D′. Guiding defects in the extension of these fibers to outer hair cells are obvious in conditional Prox1 flox/flox; Tg(Pax2-Cre) mutant littermates in the middle turn. In this case, fibers follow a predominantly radial path with random turns toward the apex and the base. Further comparison with wildtype (E, E′, E″) and FGFR3 null mutant mice (F) shows the level of disorganization more clearly (G). FGFR3 mutants have disorganized supporting cells much like the _Prox1_-null mice but clearly do not show an equally severe disorganization of afferent growth (compare F with G. Bar, 20 µm.
Figure 6. Organization of hair cells and supporting cells is mildly disrupted in Prox1 flox/flox;Pax2-Cre conditional mutant embryos.
This image shows near radial thick (A,B) and ultrathin (C–F) sections through the middle turn of a Prox1flox/flox control and a Prox1flox/flox; Pax2-Cre conditional mutant animal. Note that the overall organization into 4 rows of hair cells (one inner and three outer) and five rows of supporting cells surrounding outer hair cells (two rows of pillar and three rows of Deiter's cells) is preserved in the conditional mutant (B,D,F). However, closer examination reveals that the regular organization of hair cells and supporting cells with two heads of pillar cells between inner and first row of outer hair cells (A,C,E) is only partially conserved in conditional mutants. In fact occasionally only a single pillar cell is found between inner and outer hair cells that appears to be the outer pillar cell (D,F). Hair cells develop normal with respect to apical kinocilia and stereocilia polarity and development (arrows in E,F). Abbreviations: D1–D3, first to third row of Deiter's cells; IHC, inner hair cell; IP, inner pillar cell; OHC, outer hair cell; OP, outer pillar cell. Bar indicates 100 µm in A,B and 10 µm in D–F).
Figure 7. Hair cell differentiation is not required for Prox1 expression.
A, B. Prox1 expression is maintained in undifferentiated supporting cells of E18.5 _Atoh1_- null embryos. This result argues that Prox1 expression is independent of hair cell mediated differentiation of sensory epithelia. A′ shows the Prox1 immunostaining in the apex. Abbreviations: AC, anterior crista; HC, horizontal crista; U, utricle. Bar, 100 µm.
Figure 8. Triple immunolabeling reveals cellular and fiber disorganization in the organ of Corti of Prox1 flox/flox; Tg(Pax2-Cre) conditional mutant embryos.
Whole mount antibody staining of the organ of Corti highlighting the hair cells (anti-BDNF, blue), supporting cells (anti-Sox2, green) and nerve fibers (anti-β-tubulin, red). (A–C) The top row shows all three immunostaining together, the middle shows nerve fibers and supporting cells, and the bottom one nerve fibers and the hair cells. In contrast to the wild-type condition (A, A′ A″), in Pax2-Cre;Prox1 flox/flox conditional mutant embryos fibers extend beyond the first row of Deiter's cells (B′,C′) where they turn randomly toward the base or apex. In addition, hair cells are not in close proximity to the nerve fibers (A″, B″, C″). D1–D3− Deiter's cells, IP-inner Pillar cell, OP-outer Pillar cell, IHC-Inner hair cell, OHC1–3-outer hair cells. Bar, 100 µm.
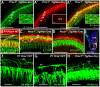
Figure 9. Immunolabeling and dye tracing reveals Type II fiber disorganization in the organ of Corti of Prox1 flox/flox; Tg(Nes-Cre) conditional mutant embryos.
(A, A′,A″) This 1 day old basal turn shows that the Prox1 protein is present in the supporting cells (A′, A″) and that neuron processes extend beyond the first row of Deiter's cells to form a bundle of intertwined fibers near the second and third row of Prox1 positive Deiter's cells. Inserts in A, A′ and A″ show tubulin immunostaining in spiral ganglion cells (SPG) but show no immunoreaction for Prox1. The disorganization of nerve fibers becomes particularly obvious in a side by side comparison with the regular pattern of cells (shown with Hoechst stain) Type II process in wildtype (B,C). In the apex, Type II fibers extend in a random way towards base and apex between Prox1 positive supporting cells (D). Epoxy section of Prox1 (red) and tubulin immunostained (green) and Hoechst counterstained (blue) organ of Corti shows the normal organization of the greater epithelial ridge (GER) with Prox1 being restricted to 5 rows of supporting cells. Point applications of lipophilic dyes allows imaging the growth cones and their regular turns toward the base in wildtype (F,G) but shows a disorganized outgrowth and growth cones (GC) in Prox1 flox/flox; Nes-Cre conditional mutant mice. D1–3, Deiter's cells row 1–3; IP, inner pillar; OP, outer pillar; SPG, spiral ganglion. Bar, 100 µm (A–D), 50 µm (E–H; inserts in A,A′, A″).
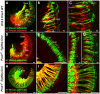
Figure 10. Dye tracing reveals Type II fiber outgrowth problems in the organ of Corti of Prox1 flox/flox; Tg(Nes-Cre) conditional mutant embryos.
NV Maroon (green) was inserted into the cochlear nucleus and NV Orange (red) was inserted into the olivocochlear bundle to label a small population of afferents (green) and all efferents (red). Efferents show a similarly organized intraganglionic spiral bundles in wildtype (A–C) and Prox1 flox/flox; Nes-Cre conditional mutant mice (D–J) and grow toether with afferents in radial fiber bundles (RF) to the organ of Corti. Note that at this stage only occasional efferents extent to outer hair cells. In contrast, type II afferents grow to the second or third row of outer hair cells (OHC) where they invariably turn toward the base (B,C). At this stage, none of the multiple type II afferents of Prox1 flox/flox; Nes-Cre conditional mutant mice show this coordinated growth pattern. Instead, fibers grow randomly toward the base or apex but mostly seem to stall with multiple branches extending toward the base and the apex (F,G). IGSB, intraganglionic spiral bundle; OHC, outer hair cells; RF, radial fibers; SPG, spiral ganglion. Bar, 100 µm (A–D), 50 µm (E–H; inserts in A,A′, A″).
Similar articles
- Expression of Prox1 during mouse cochlear development.
Bermingham-McDonogh O, Oesterle EC, Stone JS, Hume CR, Huynh HM, Hayashi T. Bermingham-McDonogh O, et al. J Comp Neurol. 2006 May 10;496(2):172-86. doi: 10.1002/cne.20944. J Comp Neurol. 2006. PMID: 16538679 Free PMC article. - Prox1 interacts with Atoh1 and Gfi1, and regulates cellular differentiation in the inner ear sensory epithelia.
Kirjavainen A, Sulg M, Heyd F, Alitalo K, Ylä-Herttuala S, Möröy T, Petrova TV, Pirvola U. Kirjavainen A, et al. Dev Biol. 2008 Oct 1;322(1):33-45. doi: 10.1016/j.ydbio.2008.07.004. Epub 2008 Jul 9. Dev Biol. 2008. PMID: 18652815 - Localization of efferent neurotransmitters in the inner ear of the homozygous Bronx waltzer mutant mouse.
Kong WJ, Scholtz AW, Hussl B, Kammen-Jolly K, Schrott-Fischer A. Kong WJ, et al. Hear Res. 2002 May;167(1-2):136-55. doi: 10.1016/s0378-5955(02)00382-9. Hear Res. 2002. PMID: 12117537 - Anatomical and physiological development of the human inner ear.
Lim R, Brichta AM. Lim R, et al. Hear Res. 2016 Aug;338:9-21. doi: 10.1016/j.heares.2016.02.004. Epub 2016 Feb 18. Hear Res. 2016. PMID: 26900072 Review. - Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain.
Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Fritzsch B, et al. Hear Res. 2005 Aug;206(1-2):52-63. doi: 10.1016/j.heares.2004.11.025. Hear Res. 2005. PMID: 16080998 Free PMC article. Review.
Cited by
- Neurod1 regulates survival and formation of connections in mouse ear and brain.
Jahan I, Kersigo J, Pan N, Fritzsch B. Jahan I, et al. Cell Tissue Res. 2010 Jul;341(1):95-110. doi: 10.1007/s00441-010-0984-6. Epub 2010 May 30. Cell Tissue Res. 2010. PMID: 20512592 Free PMC article. - Effects of 3,3'-Iminodipropionitrile on Hair Cell Numbers in Cristae of CBA/CaJ and C57BL/6J Mice.
Wilkerson BA, Artoni F, Lea C, Ritchie K, Ray CA, Bermingham-McDonogh O. Wilkerson BA, et al. J Assoc Res Otolaryngol. 2018 Oct;19(5):483-491. doi: 10.1007/s10162-018-00687-y. Epub 2018 Aug 31. J Assoc Res Otolaryngol. 2018. PMID: 30171385 Free PMC article. - Age-Related Hearing Loss: Sensory and Neural Etiology and Their Interdependence.
Elliott KL, Fritzsch B, Yamoah EN, Zine A. Elliott KL, et al. Front Aging Neurosci. 2022 Feb 17;14:814528. doi: 10.3389/fnagi.2022.814528. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35250542 Free PMC article. Review. - Sequential Retraction Segregates SGN Processes during Target Selection in the Cochlea.
Druckenbrod NR, Goodrich LV. Druckenbrod NR, et al. J Neurosci. 2015 Dec 9;35(49):16221-35. doi: 10.1523/JNEUROSCI.2236-15.2015. J Neurosci. 2015. PMID: 26658872 Free PMC article. - Rac1 and Nectin3 are essential for planar cell polarity-directed axon guidance in the peripheral auditory system.
Clancy S, Xie N, Eluvathingal Muttikkal T, Wang J, Adylkhan A, Fateh E, Smith M, Wilson P, Smith M, Hogan A, Sutherland A, Lu X. Clancy S, et al. Development. 2025 Apr 15;152(8):dev204423. doi: 10.1242/dev.204423. Epub 2025 Apr 24. Development. 2025. PMID: 40207531
References
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. - PubMed
- Kelley MW. Hair cell development: Commitment through differentiation. Brain Res 2006 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous