Vaccination targeting surface FomA of Fusobacterium nucleatum against bacterial co-aggregation: Implication for treatment of periodontal infection and halitosis - PubMed (original) (raw)
Vaccination targeting surface FomA of Fusobacterium nucleatum against bacterial co-aggregation: Implication for treatment of periodontal infection and halitosis
Pei-Feng Liu et al. Vaccine. 2010.
Abstract
The mechanical therapy with multiple doses of antibiotics is one of modalities for treatment of periodontal diseases. However, treatments using multiple doses of antibiotics carry risks of generating resistant strains and misbalancing the resident body flora. We present an approach via immunization targeting an outer membrane protein FomA of Fusobacterium nucleatum (F. nucleatum), a central bridging organism in the architecture of oral biofilms. Neutralization of FomA considerably abrogated the enhancement of bacterial co-aggregation, biofilms and production of volatile sulfur compounds mediated by an inter-species interaction of F. nucleatum with Porphyromonas gingivalis (P. gingivalis). Vaccination targeting FomA also conferred a protective effect against co-infection-induced gum inflammation. Here, we advance a novel infectious mechanism by which F. nucleatum co-opts P. gingivalis to exacerbate gum infections. FomA is highlighted as a potential target for development of new therapeutics against periodontal infection and halitosis in humans.
Published by Elsevier Ltd.
Figures
Fig. 1
The co-aggregation of F. nucleatum with P. gingivalis and biofilm enhancement. The detection of bacterial co-aggregation and biofilm formation is described in “Materials and methods”. (A) F. nucleatum (F.n), P. gingivalis (P.g), and F. nucleatum co-aggregated with P. gingivalis (F.n + P.g) were visualized using light microscopy at a magnification of 60×. Bars = 2.5 μm. (B) A Malvern Zetasizer Nano-ZS with a DLS technique was used for measuring the particle sizes of F. nucleatum (F.n), P. gingivalis (P.g), and F. nucleatum co-aggregated with P. gingivalis (F.n + P.g). (C) Compared to F. nucleatum alone (F.n) and P. gingivalis alone (P.g), biofilms were significantly enhanced when F. nucleatum was co-cultured with P. gingivalis (F.n + P.g). Bars = 3 mm.
Fig. 2
Expression, purification, and identification of recombinant FomA. A pEcoli-6×HN-GFPuv vector was inserted with a full PCR amplified FomA gene of F. nucelatum (ATCC 10953). (A) FomA was expressed in E. coli in the absence (-) or presence (+) of 1 mM IPTG. After IPTG induction, FomA (arrows) was successfully expressed in E. coli and shown at approximately 40 kDa on a 10% SDS-PAGE. (B) The recombinant FomA with a 6×HN tag was purified with a Ni-NTA column according to the manufacturer's instructions (Qiagen, Chatsworth, CA). (C) The identity of F. nucelatum FomA was validated by Nano-LC-LTQ-MS analysis (Thermo Electron Corp. Waltham, MA). Twenty eight internal peptides of FomA were fully sequenced. An internal sequence (VVEYVEKPVIVYR) of FomA corresponding to the amino acids 34 to 46 is presented.
Fig. 3
Suppression of bacterial co-aggregation and biofilm formation using neutralizing antibody to FomA. (A) ICR mice were immunized with UV-irradiated E.coli BL21(DE3) FomA or GFP for nine weeks (three boosts at a three-week interval). Twenty five μg of recombinant FomA was separated via a 10% SDS-PAGE, transferred to a PVDF membrane and reacted with anti-GFP (Anti-GFP) or anti-FomA (Anti-FomA) serum (1:1,000 dilution). Antibodies to FomA (arrow) were detected in mice immunized with E. coli BL21(DE3) FomA, but not GFP. A representative of three separate experiments with similar results was shown. (B) For neutralization, F. nucleatum was pre-incubated with 2.5% (v/v) anti-FomA (Anti-FomA) or anti-GFP serum (Anti-GFP) for 2 h, after complement was inactivated by heating at 65°C for 30 min. The neutralized F. nucleatum (4 × 108
CFU
) was mixed with P. gingivalis (103 CFU) in the ratio of 4 × 105 for 3 h and 36 h for co-aggregation and biofilm assays, respectively. After neutralization with anti-FomA or anti-GFP serum, the change in the particle sizes of co-aggregated bacteria was measured by a Malvern Zetasizer Nano-ZS. (C) Biofilms were detected after treatments of F. nucleatum (F.n), P. gingivalis (P.g) and F. nucleatum plus P. gingivalis (F.n + P.g) with in anti-FomA (Anti-FomA) or anti-GFP serum (Anti-GFP). Bars = 3 mm.
Fig. 4
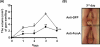
Abrogation of bacteria-induced gum swelling via passive neutralization of FomA. After pre-treatment of F. nucleatum (4 × 108 CFU) with anti-FomA or anti-GFP serum, bacteria were incubated with P. gingivalis (103 CFU) in PBS for 3 h. Both bacteria (100 μl) were subsequently administrated into gum tissues and oral cavities of ICR mice for 3 days to induce abscesses as described in “Materials and methods”. (A) The volumes of swollen gums administered with P. gingivalis plus F. nucleatum pre-treated with anti-FomA (solid circles) or anti-GFP (open circles) serum were recorded every day for 4 days. The data represent as mean ± SE (n = 4, *p<0.05, **p<0.005, ***p<0.0005 by Student's _t_-test). (B) The morphologies of swollen (circles) gums administered with P. gingivalis plus F. nucleatum pre-treated with anti-FomA (Anti-FomA) or anti-GFP serum (Anti-GFP) on the day 3 after recording were shown. Bar = 2.2 mm.
Fig. 5
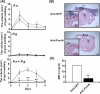
Vaccination with FomA against oral bacteria-induced gum inflammation. A gum pocket model [25] with abscesses and swollen tissues was used for evaluation of the in vivo efficacy of vaccination. (A) After inoculation with live bacteria F. nucleatum (4 × 108 CFU) (F.n), P. gingivalis (103 CFU) (P.g) or F. nucleatum plus P. gingivalis (4 × 108 CFU plus 103 CFU) (F.n + P.g) for 3 days, the severity of gum swelling (mm3) in the mice immunized with E. coli BL21(DE3) FomA (solid circles) or GFP (open circles) was measured daily for 4 days. (B) The H&E-stained gum tissue sections of lower incisors [magnification 4× and 20× (inserted panels)] displayed gum inflammation as indicated by an increase in the thickness of oral epithelium (OE) and gramulomatous reaction (arrows) in the mice immunized with E. coli BL21(DE3) GFP (Anti-GFP). Gum inflammation was significantly suppressed in mice immunized with E. coli BL21(DE3) FomA (Anti-FomA). D: dentin. Bars = 1 mm. Bars (inserted panels) = 0.2 mm. (C) Compared to vaccination with E. coli BL21(DE3) GFP (Anti-GFP), the bacteria-induced MIP-2 production was significantly diminished by vaccination with E.coli BL21(DE3) FomA (Anti-FomA). Data represent mean ± SE of five separate experiments (**p< 0.005 by Student's _t_-test).
Fig. 6
Blockage of VSC production using neutralizing antibodies to FomA. Detection of VSC production of F. nucleatum (F.n), P. gingivalis (P.g) and F. nucleatum plus P. gingivalis (F.n + P.g) in the absence (A) or presence of (B) anti-FomA or anti-GFP serum. VSC production by bacteria on the OHO-C agar plates is described in “Materials and methods”. Bars = 1.5 cm.
Similar articles
- Halitosis vaccines targeting FomA, a biofilm-bridging protein of fusobacteria nucleatum.
Liu PF, Huang IF, Shu CW, Huang CM. Liu PF, et al. Curr Mol Med. 2013 Sep;13(8):1358-67. doi: 10.2174/15665240113139990063. Curr Mol Med. 2013. PMID: 23865430 Review. - The protective effect of recombinant FomA-expressing Lactobacillus acidophilus against periodontal infection.
Ma L, Ding Q, Feng X, Li F. Ma L, et al. Inflammation. 2013 Oct;36(5):1160-70. doi: 10.1007/s10753-013-9651-x. Inflammation. 2013. PMID: 23644821 Free PMC article. - A novel vaccine targeting Fusobacterium nucleatum against abscesses and halitosis.
Liu PF, Haake SK, Gallo RL, Huang CM. Liu PF, et al. Vaccine. 2009 Mar 4;27(10):1589-95. doi: 10.1016/j.vaccine.2008.12.058. Epub 2009 Jan 20. Vaccine. 2009. PMID: 19162109 Free PMC article. - Biochemical characterization of a recombinant Lactobacillus acidophilus strain expressing exogenous FomA protein.
Ma L, Li F, Zhang X, Feng X. Ma L, et al. Arch Oral Biol. 2018 Aug;92:25-31. doi: 10.1016/j.archoralbio.2018.04.016. Epub 2018 Apr 30. Arch Oral Biol. 2018. PMID: 29747062 - Immunological Pathways Triggered by Porphyromonas gingivalis and Fusobacterium nucleatum: Therapeutic Possibilities?
de Andrade KQ, Almeida-da-Silva CLC, Coutinho-Silva R. de Andrade KQ, et al. Mediators Inflamm. 2019 Jun 24;2019:7241312. doi: 10.1155/2019/7241312. eCollection 2019. Mediators Inflamm. 2019. PMID: 31341421 Free PMC article. Review.
Cited by
- Biofilms and core pathogens shape the tumor microenvironment and immune phenotype in colorectal cancer.
Kvich L, Fritz BG, Zschach H, Terkelsen T, Raskov H, Høst-Rasmussen K, Jakobsen MR, Gheorghe AG, Gögenur I, Bjarnsholt T. Kvich L, et al. Gut Microbes. 2024 Jan-Dec;16(1):2350156. doi: 10.1080/19490976.2024.2350156. Epub 2024 May 10. Gut Microbes. 2024. PMID: 38726597 Free PMC article. - Fn-Dps, a novel virulence factor of Fusobacterium nucleatum, disrupts erythrocytes and promotes metastasis in colorectal cancer.
Wu Y, Guo S, Chen F, Li Y, Huang Y, Liu W, Zhang G. Wu Y, et al. PLoS Pathog. 2023 Jan 24;19(1):e1011096. doi: 10.1371/journal.ppat.1011096. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36693067 Free PMC article. - Oral Pathobiont-Derived Outer Membrane Vesicles in the Oral-Gut Axis.
Catalan EA, Seguel-Fuentes E, Fuentes B, Aranguiz-Varela F, Castillo-Godoy DP, Rivera-Asin E, Bocaz E, Fuentes JA, Bravo D, Schinnerling K, Melo-Gonzalez F. Catalan EA, et al. Int J Mol Sci. 2024 Oct 17;25(20):11141. doi: 10.3390/ijms252011141. Int J Mol Sci. 2024. PMID: 39456922 Free PMC article. Review. - Fusobacterium nucleatum extracellular vesicles are enriched in colorectal cancer and facilitate bacterial adhesion.
Zheng X, Gong T, Luo W, Hu B, Gao J, Li Y, Liu R, Xie N, Yang W, Xu X, Cheng L, Zhou C, Yuan Q, Huang C, Peng X, Zhou X. Zheng X, et al. Sci Adv. 2024 Sep 20;10(38):eado0016. doi: 10.1126/sciadv.ado0016. Epub 2024 Sep 20. Sci Adv. 2024. PMID: 39303027 Free PMC article. - Intestinal biofilms: pathophysiological relevance, host defense, and therapeutic opportunities.
Jandl B, Dighe S, Gasche C, Makristathis A, Muttenthaler M. Jandl B, et al. Clin Microbiol Rev. 2024 Sep 12;37(3):e0013323. doi: 10.1128/cmr.00133-23. Epub 2024 Jul 12. Clin Microbiol Rev. 2024. PMID: 38995034 Review.
References
- Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38:204–11. - PubMed
- Yaegaki K, Sanada K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J Periodontal Res. 1992;27(4 Pt 1):233–8. - PubMed
- Scully C, Rosenberg M. Halitosis. Dent Update. 2003;30(4):205–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R41 AR056169-01/AR/NIAMS NIH HHS/United States
- R21-I58002-01/PHS HHS/United States
- R01 AI067395-05/AI/NIAID NIH HHS/United States
- 1R41AR056169-01/AR/NIAMS NIH HHS/United States
- R01-AI067395-01/AI/NIAID NIH HHS/United States
- R41 AR056169/AR/NIAMS NIH HHS/United States
- R21-R022754-01/PHS HHS/United States
- R01 AI067395/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical