Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC - PubMed (original) (raw)
Comparative Study
Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC
Daejong Jeon et al. Nat Neurosci. 2010 Apr.
Abstract
Fear can be acquired vicariously through social observation of others suffering from aversive stimuli. We found that mice (observers) developed freezing behavior by observing other mice (demonstrators) receive repetitive foot shocks. Observers had higher fear responses when demonstrators were socially related to themselves, such as siblings or mating partners. Inactivation of anterior cingulate cortex (ACC) and parafascicular or mediodorsal thalamic nuclei, which comprise the medial pain system representing pain affection, substantially impaired this observational fear learning, whereas inactivation of sensory thalamic nuclei had no effect. The ACC neuronal activities were increased and synchronized with those of the lateral amygdala at theta rhythm frequency during this learning. Furthermore, an ACC-limited deletion of Ca(v)1.2 Ca(2+) channels in mice impaired observational fear learning and reduced behavioral pain responses. These results demonstrate the functional involvement of the affective pain system and Ca(v)1.2 channels of the ACC in observational social fear.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
Figure 1
Observational fear learning in the mouse. (a) Diagram of the apparatus used for observational fear conditioning and the scheme of the behavioral assay. (b,c) Observational fear learning in the mouse (nonsiblings) using a transparent (n = 21) or opaque (n = 8) partition. A significant difference in the level of freezing behavior was apparent depending on whether a transparent or an opaque partition was used for the conditioning experiment on both the training day (b) and 24 h after training (c). *P < 0.01, Scheffe’s post hoc test. (d,e) Observational fear learning with siblings. We examined freezing behavior on the day of training (_F_1, 45 = 9.41, P = 0.0036, two-way repeated ANOVA, d) and 24 h after training (_F_1, 45 = 11.48, P = 0.0015, two-way repeated ANOVA, e) in siblings (n = 26) and nonsiblings (n = 21) using a transparent partition. *P < 0.05, **P < 0.01, Scheffe’s post hoc test. Error bars represent s.e.m.
Figure 2
Observational fear learning with female mating partners as demonstrators: effect of the duration of co-housing period (familiarity). (a,b) Observational fear conditioning after 1 week of co-housing (couple, n = 10; noncouple, n = 10). There was no difference in the observational fear response (a) and the 24-h contextual memory (b) between couple and noncouple experiments. (c,d) Observational fear conditioning after a 4–5-week co-housing period (couple, n = 6; noncouple, n = 9). There was no difference in the observational training (c) and the 24-h contextual memory (d) between couple and noncouple experiments. (e,f) Observational fear conditioning after 10–15 weeks of co-housing (couple, n = 12; noncouple, n = 7). There were significant differences in the observational training (_F_1,17 = 11.41, P = 0.0036, two-way repeated ANOVA, e) and the 24-h contextual memory (_F_1,17 = 11.77, P = 0.0032, two-way repeated ANOVA, f) between couple and noncouple experiments. (g,h) Observational fear conditioning after 20–36 weeks of co-housing (couple, n = 9; noncouple, n = 7). There were significant differences in the observational training (_F_1,14 = 8.62, P = 0.0109, two-way repeated ANOVA, g) and the 24-h contextual memory (_F_1,14 = 17.21, P = 0.001, two-way repeated ANOVA, h) between couple and noncouple experiments. (i) The strength of the fear response was increased with as the duration of the co-housing periods increased. ANOVA (_F_3,33 = 3.38, P = 0.029) of the total freezing time revealed a graded effect of the duration of co-housing period on the development of observational fear and there was a significant difference in total freezing time between 1-week co-housing period group and 10–15-week or 20–36-week groups. *P < 0.05, **P < 0.01, Scheffe’s post hoc test. Error bars represent s.e.m.
Figure 3
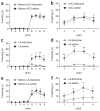
The ACC and MITN are involved in observational fear learning. (a) Mice with lidocaine injections into the ACC (n = 12) before training failed to acquire fear compared with those receiving saline injections (n = 11) (_F_1, 21 = 19.20, P = 0.0003, two-way repeated ANOVA). (b) Contextual memory 24 h after the training in a (_F_1, 21 = 16.43, P = 0.0006, two-way repeated ANOVA). (c) Mice with lidocaine injections into the ACC did not efficiently acquire fear by observation of siblings and mating partners (couples; _F_1, 30 = 18.25, P = 0.0002, two-way repeated ANOVA). (d,e) Administration of lidocaine into the parafascicular (PF) thalamic nuclei (n = 8) before training led to impaired observational fear learning during training (_F_1, 15 = 43.84, P < 0.0001, two-way repeated ANOVA, d) and 24 h after training (_F_1, 15 = 8.55, P = 0.0105, two-way repeated ANOVA, e) as compared with those receiving saline injections (n = 9). (f,g) Administration of lidocaine into the mediodorsal (MD) thalamic nuclei (n = 12) before training caused impaired observational fear learning during training (_F_1, 28 = 24.11, P < 0.0001, two-way repeated ANOVA, f) and 24 h after training (_F_1, 28 = 5.19, P = 0.0306, two-way repeated ANOVA, g) as compared with those receiving saline injections (n = 18). (h,i) Administration of lidocaine into the VPL/VPM before training had no influence on the acquisition of observational fear (h) and 24-h contextual memory (i) (lidocaine, n = 10; saline, n = 12). *P < 0.05, **P < 0.01, Scheffe’s post hoc test. Error bars represent s.e.m.
Figure 4
The ACC is involved in the acquisition of observational fear, but not in memory retrieval of observational fear and in classical fear conditioning. (a) Mice (n = 9) were trained with observational fear learning. (b) Local inactivation of the ACC 8 min before the 24-h contextual memory test did not affect the expression of fear in observational fear–conditioned mice as compared with fear expression by saline-injected mice (n = 14) (_F_1, 21 = 0.001, P = 0.99, two-way repeated ANOVA). (c,d) The contribution of the lateral amygdala (LA) to observational fear conditioning. Mice with lidocaine injections into the ACC (n = 8) before training failed to acquire fear compared with those receiving saline injections (n = 10) (_F_1, 16 = 11.46, P = 0.004, two-way repeated ANOVA, c); the same was true for contextual memory 24 h after training (_F_1, 16 = 21.34, P = 0.0003, two-way repeated ANOVA, d). (e,f) Local inactivation of the lateral amygdala (n = 8) before the 24-h contextual memory test (f) disrupted the expression of fear in observational fear-conditioned mice (e), as compared with fear shown by saline-injected mice (n = 7) (_F_1, 13 = 7.66, P = 0.016, two-way repeated ANOVA). *P < 0.05, **P < 0.01, Scheffe’s post hoc test. Error bars represent s.e.m.
Figure 5
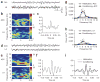
Synchronized theta activity between the ACC and lateral amygdala during learning of fear by observation. (a) Representative original traces of field potential recordings (8 s) in the ACC (upper) and lateral amygdala (bottom) during habituation. (b) Colored power spectra of the traces shown in a. (c) Cross-correlation analysis revealed no correlated neuronal activity in the two brain areas. (d) Representative original traces of field potential recordings in the ACC (upper) and lateral amygdala (bottom) during training. (e) Colored power spectra of the traces shown in d. Note the increased theta rhythms at 4–7 Hz. (f) Cross-correlation analysis revealed correlated neuronal activities in the two brain areas. (g,h) Averaged power spectra of neuronal activities (n = 7) in the ACC (g) and lateral amygdala (h) taken over an 8-s period just before delivery of the first foot shock (habituation) and after the last foot shock (conditioning). *P < 0.05, one-way ANOVA. (i) Averaged cross-correlograms of neuronal activities in the ACC and lateral amygdala taken over an 8-s period just before delivery of the first foot shock (habituation) and after the last foot shock (conditioning) (n = 7). ** indicates a significant difference in the amplitude of the second peaks between the two (P < 0.05, Student’s t test).
Figure 6
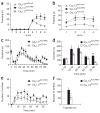
Cav1.2ACC/Cre mice showed impaired observational fear learning and reduced pain responses. (a,b) Observational fear conditioning of Cav1.2ACC/Cre (n = 22), Cav1.2ACC/PBS (n = 22) and Cav1.2loxP/loxP (n = 13) mice. Similar freezing levels were seen during training (_F_1, 33 = 0.48, P = 0.49, two-way repeated ANOVA) and in the 24-h contextual memory test (_F_1,33 = 0.95, P = 0.34, two-way repeated ANOVA) between Cav1.2ACC/PBS (PBS injected) and Cav1.2loxP/loxP (non-injected) observers, and the results were pooled for analysis. The Cav1.2ACC/Cre observers exhibited impaired observational fear learning during training (_F_1, 55 = 17.47, P < 0.0001, two-way repeated ANOVA, a) and 24-h contextual memory (_F_1, 55 = 20.85, P < 0.0001, b). *P < 0.01, Scheffe’s post hoc test. (c,d) Reduced inflammatory pain responses to formalin in Cav1.2ACC/Cre mice. Behavioral responses to a formalin injection, plotted in 5-min intervals, in Cav1.2ACC/PBS mice (n = 9) compared with Cav1.2ACC/Cre mice (n = 15) are shown in c. Data from c were grouped into five time intervals (d). *P < 0.05, **P < 0.0001, one-way ANOVA. (e,f) Reduced behavioral responses to acetic acid–induced visceral pain in Cav1.2ACC/Cre mice. Behavioral responses to acetic acid, plotted in 5-min intervals, in Cav1.2ACC/PBS mice (n = 7) and Cav1.2ACC/Cre mice (n = 6) are shown in e. The total numbers of writhing events over 60 min are shown in f. *P < 0.05, one-way ANOVA. Error bars represent s.e.m.
Comment in
- Mouse brains wired for empathy?
Grenier F, Lüthi A. Grenier F, et al. Nat Neurosci. 2010 Apr;13(4):406-8. doi: 10.1038/nn0410-406. Nat Neurosci. 2010. PMID: 20348937 No abstract available.
Similar articles
- Neural Basis of Observational Fear Learning: A Potential Model of Affective Empathy.
Keum S, Shin HS. Keum S, et al. Neuron. 2019 Oct 9;104(1):78-86. doi: 10.1016/j.neuron.2019.09.013. Neuron. 2019. PMID: 31600517 Review. - Cortical representations of affective pain shape empathic fear in male mice.
Choi J, Lee YB, So D, Kim JY, Choi S, Kim S, Keum S. Choi J, et al. Nat Commun. 2025 Feb 24;16(1):1937. doi: 10.1038/s41467-025-57230-w. Nat Commun. 2025. PMID: 39994222 Free PMC article. - Predicting aversive events and terminating fear in the mouse anterior cingulate cortex during trace fear conditioning.
Steenland HW, Li XY, Zhuo M. Steenland HW, et al. J Neurosci. 2012 Jan 18;32(3):1082-95. doi: 10.1523/JNEUROSCI.5566-11.2012. J Neurosci. 2012. PMID: 22262906 Free PMC article. - Roles of mediodorsal thalamus in observational fear-related neural activity in mouse anterior cingulate cortex.
Ramesh K, Nair IR, Yamamoto N, Ogawa SK, Terranova JI, Kitamura T. Ramesh K, et al. Mol Brain. 2025 Feb 25;18(1):14. doi: 10.1186/s13041-025-01188-9. Mol Brain. 2025. PMID: 40001162 Free PMC article. - Interplay of amygdala and cingulate plasticity in emotional fear.
Toyoda H, Li XY, Wu LJ, Zhao MG, Descalzi G, Chen T, Koga K, Zhuo M. Toyoda H, et al. Neural Plast. 2011;2011:813749. doi: 10.1155/2011/813749. Epub 2011 Sep 7. Neural Plast. 2011. PMID: 21912749 Free PMC article. Review.
Cited by
- Anterior Cingulate Cortex Signals Attention in a Social Paradigm that Manipulates Reward and Shock.
Schneider KN, Sciarillo XA, Nudelman JL, Cheer JF, Roesch MR. Schneider KN, et al. Curr Biol. 2020 Oct 5;30(19):3724-3735.e2. doi: 10.1016/j.cub.2020.07.039. Epub 2020 Aug 6. Curr Biol. 2020. PMID: 32763169 Free PMC article. - Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress.
Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Hanke ML, et al. Brain Behav Immun. 2012 Oct;26(7):1150-9. doi: 10.1016/j.bbi.2012.07.011. Epub 2012 Jul 24. Brain Behav Immun. 2012. PMID: 22841997 Free PMC article. - Comprehensive behavioral analysis of voltage-gated calcium channel beta-anchoring and -regulatory protein knockout mice.
Nakao A, Miki T, Shoji H, Nishi M, Takeshima H, Miyakawa T, Mori Y. Nakao A, et al. Front Behav Neurosci. 2015 Jun 16;9:141. doi: 10.3389/fnbeh.2015.00141. eCollection 2015. Front Behav Neurosci. 2015. PMID: 26136667 Free PMC article. - Pathogens, odors, and disgust in rodents.
Kavaliers M, Ossenkopp KP, Choleris E. Kavaliers M, et al. Neurosci Biobehav Rev. 2020 Dec;119:281-293. doi: 10.1016/j.neubiorev.2020.09.037. Epub 2020 Oct 6. Neurosci Biobehav Rev. 2020. PMID: 33031813 Free PMC article. Review. - Clinical Therapeutic Strategy and Neuronal Mechanism Underlying Post-Traumatic Stress Disorder (PTSD).
Yabuki Y, Fukunaga K. Yabuki Y, et al. Int J Mol Sci. 2019 Jul 24;20(15):3614. doi: 10.3390/ijms20153614. Int J Mol Sci. 2019. PMID: 31344835 Free PMC article. Review.
References
- Olsson A, Phelps EA. Social learning of fear. Nat Neurosci. 2007;10:1095–1102. - PubMed
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. - PubMed
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. - PubMed
- Adolphs R. Cognitive neuroscience of human social behavior. Nat Rev Neurosci. 2003;4:165–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous