Membrane transporters in drug development - PubMed (original) (raw)
Review
doi: 10.1038/nrd3028.
Kathleen M Giacomini, Shiew-Mei Huang, Donald J Tweedie, Leslie Z Benet, Kim L R Brouwer, Xiaoyan Chu, Amber Dahlin, Raymond Evers, Volker Fischer, Kathleen M Hillgren, Keith A Hoffmaster, Toshihisa Ishikawa, Dietrich Keppler, Richard B Kim, Caroline A Lee, Mikko Niemi, Joseph W Polli, Yuichi Sugiyama, Peter W Swaan, Joseph A Ware, Stephen H Wright, Sook Wah Yee, Maciej J Zamek-Gliszczynski, Lei Zhang
- PMID: 20190787
- PMCID: PMC3326076
- DOI: 10.1038/nrd3028
Review
Membrane transporters in drug development
International Transporter Consortium et al. Nat Rev Drug Discov. 2010 Mar.
Abstract
Membrane transporters can be major determinants of the pharmacokinetic, safety and efficacy profiles of drugs. This presents several key questions for drug development, including which transporters are clinically important in drug absorption and disposition, and which in vitro methods are suitable for studying drug interactions with these transporters. In addition, what criteria should trigger follow-up clinical studies, and which clinical studies should be conducted if needed. In this article, we provide the recommendations of the International Transporter Consortium on these issues, and present decision trees that are intended to help guide clinical studies on the currently recognized most important drug transporter interactions. The recommendations are generally intended to support clinical development and filing of a new drug application. Overall, it is advised that the timing of transporter investigations should be driven by efficacy, safety and clinical trial enrolment questions (for example, exclusion and inclusion criteria), as well as a need for further understanding of the absorption, distribution, metabolism and excretion properties of the drug molecule, and information required for drug labelling.
Figures
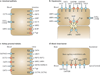
Figure 1. selected human transport proteins for drugs and endogenous substances
Transporters in plasma membrane domains of intestinal epithelia, hepatocytes, kidney proximal tubules and brain capillary endothelial cells are presented. Those coloured in red indicate that the selected transporters are described in detail in this manuscript. Those coloured in blue indicate that the transport proteins are of importance but are not described in this manuscript. a | Intestinal epithelia contain in their apical (luminal) membrane several uptake transporters including one or more members of the organic anion transporting polypeptide (OATP) family; peptide transporter 1 (PEPT1; SLC15A1); ileal apical sodium/bile acid co-transporter (ASBT; SLC10A2); and monocarboxylic acid transporter 1 (MCT1; SLC16A1). The apical ATP-dependent efflux pumps include multidrug resistance protein 2 (MRP2; ABCC2); breast cancer resistance protein (BCRP; ABCG2); and P-glycoprotein (P-gp; MDR1, ABCB1). The basolateral membrane of intestinal epithelia contains organic cation transporter 1 (OCT1; SLC22A1); heteromeric organic solute transporter (OSTα–OSTβ); and MRP3 (ABCC3). b | Human hepatocyte uptake transporters in the basolateral (sinusoidal) membrane include the sodium/taurocholate co-transporting peptide (NTCP; SLC10A1); three members of the OATP family (OATP1B1 (SLCO1B1), OATP1B3 (SLCO1B3) and OATP2B1 (SLCO2B1)); organic anion transporter 2 (OAT2; SLC22A7) and OAT7 (SLC22A9); and OCT1. Efflux pumps in the hepatocyte basolateral membrane include MRP3, MRP4 (ABCC4) and MRP6 (ABCC6). Apical (canalicular) efflux pumps of the hepatocyte comprise P-gp; bile-salt export pump (BSEP or SPGP; ABCB11); BCRP (ABCG2); and MRP2. In addition, multidrug and toxin extrusion protein 1 (MATE1; SLC47A1) is located in the apical hepatocyte membrane. c | Kidney proximal tubules contain in the apical (luminal) membrane OAT4 (SLC22A11); urate transporter 1 (URAT1; SCL22A12); PEPT1 and PEPT2 (SLC15A2); MRP2 and MRP4; MATE1 and MATE2-K (SLC47A2); P-gp; organic cation/ergothioneine transporter (OCTN1; SLC22A4); and organic cation/carnitine transporter (OCTN2; SLC22A5). Basolateral uptake transporters in proximal tubule epithelia include OATP4C1 (SLCO4C1); OCT2; and OAT1, OAT2 and OAT3 (SLC22A8). d | Apical (luminal) transport proteins of brain capillary endothelial cells contributing to the function of the blood–brain barrier include the uptake transporters OATP1A2 and OATP2B1; and the efflux pumps P-gp, BCRP, MRP4 and MRP5 (ABCC5). Note that localization of transporters to particular membranes and tissues is sometimes controversial; therefore, the International Transporter Consortium erred on the conservative side in only showing the localization of transporters for which good evidence exists.
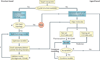
Figure 2. Decision tree for computer modelling of transporter proteins
Ligand-based methods such as pharmacophore and 3D-quantitative structure–activity relationship modelling (3D-QSAR) will be required when crystal structure templates are not available or can be used to complement existing homology models. Generally, a high-throughput in vitro assay is used to generate a data set of transporter ligands (substrates or inhibitors) and their corresponding activity values (_K_m/_J_max, _K_i, or percentage inhibition). a | Data sets are split into a training set used to construct a model and a test set to validate the model. b | Criteria for model acceptance depend on its ability to successfully predict biological activity of test set molecules. c | Acceptable models may be combined to generate synergistic consensus models. d | To generate homology models with high confidence, the membrane topology of both the target and scaffold proteins must match. Modelling of transporters with divergent topology should be attempted with great caution as it is currently unclear how the arbitrary deletion or insertion of a membrane helix may distort the overall helical packing of membrane proteins. e | To further increase fidelity, models should be optimized using molecular dynamics simulations (MDS), preferably embedded in a lipid bilayer surrounded by an aqueous environment. f | When all criteria are satisfied, homology models may be used to determine regions of substrate binding or interaction and mapping of possible permeation pathways. g | Biochemical validation — for example, substituted cysteine accessibility method (SCAM) — will therefore be needed. TMD, transmembrane domain.
Comment in
- Transporters in drug development: advancing on the Critical Path.
Huang SM, Woodcock J. Huang SM, et al. Nat Rev Drug Discov. 2010 Mar;9(3):175-6. doi: 10.1038/nrd3124. Nat Rev Drug Discov. 2010. PMID: 20222180 No abstract available. - Understanding transport through pharmacological barriers--are we there yet?
Sarkadi B, Szakács G. Sarkadi B, et al. Nat Rev Drug Discov. 2010 Nov;9(11):897-8. doi: 10.1038/nrd3187-c1. Epub 2010 Oct 29. Nat Rev Drug Discov. 2010. PMID: 21031004 No abstract available. - The relevance of assessment of intestinal P-gp inhibition using digoxin as an in vivo probe substrate.
Shi JG, Zhang Y, Yeleswaram S. Shi JG, et al. Nat Rev Drug Discov. 2011 Jan;10(1):75; author reply 75. doi: 10.1038/nrd3028-c1. Nat Rev Drug Discov. 2011. PMID: 21193869 No abstract available.
Similar articles
- In vitro methods to support transporter evaluation in drug discovery and development.
Brouwer KL, Keppler D, Hoffmaster KA, Bow DA, Cheng Y, Lai Y, Palm JE, Stieger B, Evers R; International Transporter Consortium. Brouwer KL, et al. Clin Pharmacol Ther. 2013 Jul;94(1):95-112. doi: 10.1038/clpt.2013.81. Epub 2013 Apr 10. Clin Pharmacol Ther. 2013. PMID: 23588315 Review. - Use of PET Imaging to Evaluate Transporter-Mediated Drug-Drug Interactions.
Langer O. Langer O. J Clin Pharmacol. 2016 Jul;56 Suppl 7:S143-56. doi: 10.1002/jcph.722. J Clin Pharmacol. 2016. PMID: 27385172 Review. - Evaluation of Proposed In Vivo Probe Substrates and Inhibitors for Phenotyping Transporter Activity in Humans.
Momper JD, Tsunoda SM, Ma JD. Momper JD, et al. J Clin Pharmacol. 2016 Jul;56 Suppl 7:S82-98. doi: 10.1002/jcph.736. J Clin Pharmacol. 2016. PMID: 27385182 Review. - Development of novel, 384-well high-throughput assay panels for human drug transporters: drug interaction and safety assessment in support of discovery research.
Tang H, Shen DR, Han YH, Kong Y, Balimane P, Marino A, Gao M, Wu S, Xie D, Soars MG, O'Connell JC, Rodrigues AD, Zhang L, Cvijic ME. Tang H, et al. J Biomol Screen. 2013 Oct;18(9):1072-83. doi: 10.1177/1087057113494807. J Biomol Screen. 2013. PMID: 24062352 - Transporter studies in drug development: experience to date and follow-up on decision trees from the International Transporter Consortium.
Tweedie D, Polli JW, Berglund EG, Huang SM, Zhang L, Poirier A, Chu X, Feng B; International Transporter Consortium. Tweedie D, et al. Clin Pharmacol Ther. 2013 Jul;94(1):113-25. doi: 10.1038/clpt.2013.77. Epub 2013 Apr 10. Clin Pharmacol Ther. 2013. PMID: 23588318 Review.
Cited by
- Comparison of Canine and Human Physiological Factors: Understanding Interspecies Differences that Impact Drug Pharmacokinetics.
Martinez MN, Mochel JP, Neuhoff S, Pade D. Martinez MN, et al. AAPS J. 2021 Apr 27;23(3):59. doi: 10.1208/s12248-021-00590-0. AAPS J. 2021. PMID: 33907906 Review. - A key role for the transporter OAT1 in systemic lipid metabolism.
Granados JC, Nigam AK, Bush KT, Jamshidi N, Nigam SK. Granados JC, et al. J Biol Chem. 2021 Jan-Jun;296:100603. doi: 10.1016/j.jbc.2021.100603. Epub 2021 Mar 27. J Biol Chem. 2021. PMID: 33785360 Free PMC article. - Active sulforhodamine 101 uptake into hippocampal astrocytes.
Schnell C, Hagos Y, Hülsmann S. Schnell C, et al. PLoS One. 2012;7(11):e49398. doi: 10.1371/journal.pone.0049398. Epub 2012 Nov 26. PLoS One. 2012. PMID: 23189143 Free PMC article. - Targeting ABCB1-mediated tumor multidrug resistance by CRISPR/Cas9-based genome editing.
Yang Y, Qiu JG, Li Y, Di JM, Zhang WJ, Jiang QW, Zheng DW, Chen Y, Wei MN, Huang JR, Wang K, Shi Z. Yang Y, et al. Am J Transl Res. 2016 Sep 15;8(9):3986-3994. eCollection 2016. Am J Transl Res. 2016. PMID: 27725879 Free PMC article. - A Metabolomics Approach for Predicting OATP1B-Type Transporter-Mediated Drug-Drug Interaction Liabilities.
Li Y, Jin Y, Taheri H, Schmidt KT, Gibson AA, Buck SAJ, Eisenmann ED, Mathijssen RHJ, Figg WD, Baker SD, Sparreboom A, Hu S. Li Y, et al. Pharmaceutics. 2022 Sep 13;14(9):1933. doi: 10.3390/pharmaceutics14091933. Pharmaceutics. 2022. PMID: 36145680 Free PMC article.
References
- Giacomini KM, Sugiyama Y. In: Gilman’s The Pharmacological Basis of Therapeutics. Brunton LL, Lazo JS, Parker RL, editors. New York: McGraw-Hill; 2006. pp. 41–70. This chapter in a textbook provides an excellent overview of transporters.
- Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv. Drug Deliv. Rev. 2003;55:3–29. This manuscript provides an excellent review of ABC transporters that are important in drug response.
- Sai Y. Biochemical and molecular pharmacological aspects of transporters as determinants of drug disposition. Drug Metab. Pharmacokinet. 2005;20:91–99. - PubMed
- Cascorbi I. Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol. Ther. 2006;112:457–473. - PubMed
- Choudhuri S, Klaassen CD. Structure, function, expression, genomic organization, and single nucleotide polymorphisms of human ABCB1 (MDR1), ABCC (MRP), and ABCG2 (BCRP) efflux transporters. Int. J. Toxicol. 2006;25:231–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK061425/DK/NIDDK NIH HHS/United States
- R01 GM041935/GM/NIGMS NIH HHS/United States
- P30 ES006694/ES/NIEHS NIH HHS/United States
- GM61390/GM/NIGMS NIH HHS/United States
- U01 GM061390/GM/NIGMS NIH HHS/United States
- U01 GM061390-10/GM/NIGMS NIH HHS/United States
- R01 DK061425-07/DK/NIDDK NIH HHS/United States
- R01 DK080801/DK/NIDDK NIH HHS/United States
- U19 GM061390/GM/NIGMS NIH HHS/United States
- R01 DK061425-08/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases