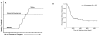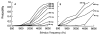Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group - PubMed (original) (raw)
Review
Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group
Satkiran Grewal et al. Pediatrics. 2010 Apr.
Abstract
Children treated for malignancies may be at risk for early- or delayed-onset hearing loss that can affect learning, communication, school performance, social interaction, and overall quality of life. Survivors at particular risk include those treated with platinum compounds (cisplatin and/or carboplatin) for neuroblastoma, hepatoblastoma, osteosarcoma, or germ-cell tumors and/or those treated with radiation that affects the ear at doses of >30 Gy for pediatric head and neck tumors. The aims of the Auditory/Hearing Late Effects Task Force of the Children's Oncology Group in this report were to (1) review ototoxicity resulting from childhood cancer therapy including platinum compounds (cisplatin and carboplatin) and radiation, (2) describe briefly cochlear pathophysiology and genetics of cisplatin-related hearing loss, (3) explain the impact of hearing loss resulting from chemotherapy and radiation, and (4) offer recommendations regarding evaluation and management of pediatric patients who are at risk for treatment-related hearing loss. A questionnaire is included as a tool to assist pediatricians in assessment.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT: The authors have indicated they have no financial relationships relative to this article to disclose
Figures
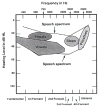
Figure 1
Frequency composition (spectra) and approximate intensity ranges, within conversational speech, for different categories of speech sounds. From Hall JW, Mueller HG (eds) Audiologist’s Desk Reference, with permission.
Figure 2
Figure 2A. Progressive hearing loss with increasing cycles of cisplatin chemotherapy with threshold effect [with permission from Schell et. al] Figure 2B. Median time to hearing loss [with permission from Knight KR et. al]
Figure 3
Sequential audiometry in a 4 year-old diagnosed with neuroblastoma: pre-chemotherapy (A); after completing treatment including 400 mg/m2 of cisplatin (B); and following full therapy with cisplatin 400 mg/m2 along with carboplatin 1,700 mg/m2 **(C).**[Modified from Knight KR et. al with permission ]
Figure 4
Probability of hearing loss at various frequencies related to cumulative dose of cisplatin. A=group that did not receive cranial irradiation, B=group that received cranial irradiation [with permission from Schell et al ]
Figure 5
Figure 6
Similar articles
- Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2014 Jul 1;(7):CD009219. doi: 10.1002/14651858.CD009219.pub3. Cochrane Database Syst Rev. 2014. PMID: 24984156 Review. - Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2012 May 16;(5):CD009219. doi: 10.1002/14651858.CD009219.pub2. Cochrane Database Syst Rev. 2012. PMID: 22592737 Updated. Review. - Platinum-induced hearing loss after treatment for childhood cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2016 Aug 3;2016(8):CD010181. doi: 10.1002/14651858.CD010181.pub2. Cochrane Database Syst Rev. 2016. PMID: 27486906 Free PMC article. Review. - Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss.
Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N, Hartmann O. Bertolini P, et al. J Pediatr Hematol Oncol. 2004 Oct;26(10):649-55. J Pediatr Hematol Oncol. 2004. PMID: 15454836 - Ototoxicity in children with high-risk neuroblastoma: prevalence, risk factors, and concordance of grading scales--a report from the Children's Oncology Group.
Landier W, Knight K, Wong FL, Lee J, Thomas O, Kim H, Kreissman SG, Schmidt ML, Chen L, London WB, Gurney JG, Bhatia S. Landier W, et al. J Clin Oncol. 2014 Feb 20;32(6):527-34. doi: 10.1200/JCO.2013.51.2038. Epub 2014 Jan 13. J Clin Oncol. 2014. PMID: 24419114 Free PMC article.
Cited by
- Modifiable Lifestyle Risk Factors in Adult Survivors of Childhood Cancer: A Nationally Representative Study.
Ton MD, Chan JSK, Satti DI, Peckham-Gregory EC, Mahal BA, Isrow D, Dee EC, Swami NS. Ton MD, et al. Am J Clin Oncol. 2024 Oct 1;47(10):485-495. doi: 10.1097/COC.0000000000001123. Epub 2024 Jun 24. Am J Clin Oncol. 2024. PMID: 38913415 - Identifying causes of balance impairment and exploring sensory contributions to balance in pediatric oncology: A scoping review.
McCarthy E, Marchese VG, Shipper AG, Rock K, Felter C. McCarthy E, et al. Crit Rev Oncol Hematol. 2024 Sep;201:104425. doi: 10.1016/j.critrevonc.2024.104425. Epub 2024 Jun 22. Crit Rev Oncol Hematol. 2024. PMID: 38909876 Review. - Remote Field Application of Digital Technology for Hearing Assessments in a Cohort of Pediatric Germ Cell Tumor Survivors.
Monterroso PS, Knight K, Roesler MA, Sample JM, Poynter JN. Monterroso PS, et al. Cancer Epidemiol Biomarkers Prev. 2024 Sep 3;33(9):1177-1184. doi: 10.1158/1055-9965.EPI-24-0203. Cancer Epidemiol Biomarkers Prev. 2024. PMID: 38869488 - St. Jude Survivorship Portal: Sharing and Analyzing Large Clinical and Genomic Datasets from Pediatric Cancer Survivors.
Matt GY, Sioson E, Shelton K, Wang J, Lu C, Zaldivar Peraza A, Gangwani K, Paul R, Reilly C, Acić A, Liu Q, Sandor SR, McLeod C, Patel J, Wang F, Im C, Wang Z, Sapkota Y, Wilson CL, Bhakta N, Ness KK, Armstrong GT, Hudson MM, Robison LL, Zhang J, Yasui Y, Zhou X. Matt GY, et al. Cancer Discov. 2024 Aug 2;14(8):1403-1417. doi: 10.1158/2159-8290.CD-23-1441. Cancer Discov. 2024. PMID: 38593228 Free PMC article. - Children with non-central nervous system tumors treated with platinum-based chemotherapy are at risk for hearing loss and cognitive impairments.
L'Hotta AJ, Spence A, Varughese TE, Felts K, Hayashi SS, Jones-White M, LaFentres E, Lieu JEC, Hayashi RJ, King AA. L'Hotta AJ, et al. Front Pediatr. 2024 Mar 20;12:1341762. doi: 10.3389/fped.2024.1341762. eCollection 2024. Front Pediatr. 2024. PMID: 38571700 Free PMC article.
References
- McHaney VA, Thibadoux G, Hayes FA, Green AA. Hearing loss in children receiving cisplatin chemotherapy. J Pediatr. 1983;102:314–317. - PubMed
- Schell MJ, McHaney VA, Green AA, et al. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. J Clin Oncol. 1989;7:754–760. - PubMed
- Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Med Pediatr Oncol. 1991;19:295–300. - PubMed
- Ilveskoski I, Saarinen UM, Wiklund T, et al. Ototoxicity in children with malignant brain tumors treated with the “8 in 1” chemotherapy protocol. Med Pediatr Oncol. 1996;27:26–31. - PubMed
- Kortmann RD, Kuhl J, Timmermann B, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT “91. Int J Radiat Oncol Biol Phys. 2000;46:269–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical