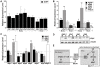Molecular evolution and functional characterization of Drosophila insulin-like peptides - PubMed (original) (raw)
Molecular evolution and functional characterization of Drosophila insulin-like peptides
Sebastian Grönke et al. PLoS Genet. 2010.
Abstract
Multicellular animals match costly activities, such as growth and reproduction, to the environment through nutrient-sensing pathways. The insulin/IGF signaling (IIS) pathway plays key roles in growth, metabolism, stress resistance, reproduction, and longevity in diverse organisms including mammals. Invertebrate genomes often contain multiple genes encoding insulin-like ligands, including seven Drosophila insulin-like peptides (DILPs). We investigated the evolution, diversification, redundancy, and functions of the DILPs, combining evolutionary analysis, based on the completed genome sequences of 12 Drosophila species, and functional analysis, based on newly-generated knock-out mutations for all 7 dilp genes in D. melanogaster. Diversification of the 7 DILPs preceded diversification of Drosophila species, with stable gene diversification and family membership, suggesting stabilising selection for gene function. Gene knock-outs demonstrated both synergy and compensation of expression between different DILPs, notably with DILP3 required for normal expression of DILPs 2 and 5 in brain neurosecretory cells and expression of DILP6 in the fat body compensating for loss of brain DILPs. Loss of DILP2 increased lifespan and loss of DILP6 reduced growth, while loss of DILP7 did not affect fertility, contrary to its proposed role as a Drosophila relaxin. Importantly, loss of DILPs produced in the brain greatly extended lifespan but only in the presence of the endosymbiontic bacterium Wolbachia, demonstrating a specific interaction between IIS and Wolbachia in lifespan regulation. Furthermore, loss of brain DILPs blocked the responses of lifespan and fecundity to dietary restriction (DR) and the DR response of these mutants suggests that IIS extends lifespan through mechanisms that both overlap with those of DR and through additional mechanisms that are independent of those at work in DR. Evolutionary conservation has thus been accompanied by synergy, redundancy, and functional differentiation between DILPs, and these features may themselves be of evolutionary advantage.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
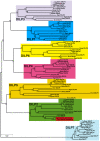
Figure 1. Phylogeny of Drosophila insulin-like peptides.
The evolutionary history of the seven DILP families from 12 fully sequenced Drosophila species inferred using the Neighbour-Joining method. Bootstrap replicate support percentages are shown next to the branches. The tree is drawn to scale, with branch lengths proportional to evolutionary distance computed using the JTT matrix-based method. 7 DILPs have remained present and clearly differentiated from each other during the more than 40 million years of evolution of Drosophilidae flies. D. grimshawi contains two DILP2 peptides (shaded in red).
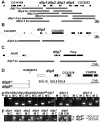
Figure 2. Gene locus organization and generation of dilp mutants.
(A–C) Null mutations for dilp1, 2, 3, 4, 5, 7, 2_–_3, and 1_–_4 were generated by ends-out homologous recombination and by imprecise P-element excision for dilp6 (D). (A) The dilp1_–_4 genes cluster at cytological position 67C8 is separated from dilp5 by two intervening genes of unknown function (Flybase: CG32052 and CG33205). (B) dilp5 is located within an intron of the CG33205 gene. (C) dilp7 is located on the X-chromosome at 3E2 immediately downstream of the essential Poly(ADP-ribose) glycohydrolase (Parg) gene. Donor constructs (dilp ko) for ends-out homologous recombination are indicated by grey bars. Gap between grey bars indicates the genomic region replaced by a whitehs marker gene. Coding parts of exons are marked in black, non-coding parts by white boxes. (D) Transposon integration line KG004972 was used to generate dilp6 deletion mutants dilp641 and dilp668. Note: both dilp6 deletion alleles contain remaining P-element sequence, hatched line: region of breakpoint in dilp641. (E) PCR on genomic DNA of dilp mutants with dilp specific primer combinations shows homologous recombination mediated replacement of dilp genes by the whitehs marker gene and deletion of dilp6 in dilp668 mutants (M: mutant, C: control). (F) RT–PCR analysis confirms that dilp mutants are transcript null alleles. dilp641 mutants express ectopic dilp6 transcripts that lack the first exon but contain the full ORF. dilp668 mutants are dilp6 transcript null alleles.
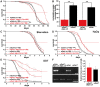
Figure 3. Compensatory regulation of gene expression in dilp mutants.
(A) Q-RT-PCR analysis of 4E-BP expression on fly bodies and fly heads of dilp single mutants. Expression of the IIS downstream target gene 4E-BP is not changed in dilp single mutants. (B) Q-RT-PCR analysis demonstrates up-regulation of 4E-BP and dilp6 and down-regulation of ImpL2 in dilp2–3,5 mutants independent of Wolbachia infection status (wDahT: _Wolbachia_-, wDah: Wolbachia+). (C) Compensatory regulation among MNC-expressed DILPs. Expression levels of DILP2, 3, and 5 were measured by QRT-PCR on RNA extracted from heads of dilp mutants. (D) Western blot analysis of DILP2 expression in total head protein confirms lack of DILP2 expression in dilp2 and dilp2_–_3 mutants and downregulation of DILP2 levels in dilp3 mutants. M: homozygous mutant, C: w1118 control. An anti-Tubulin antibody was used as loading control. (E) Diagram summarizing the feedback system involved in the control of DILP expression levels. DILP3 is part of a feedback system that acts in an autocrine/paracrine manner to regulate DILP expression in the MNC. DILP expression between MNC in the central nervous system and the peripheral fat body tissue is regulated by a negative feedback system that involves DILP6. See text for further details. Arrows denote activation, blunted lines denote repression. All experiments in (A–D) were done using 8–10 day old females reared on 1x SY-A food. Expression level of mutants were normalised to the corresponding wild type control, which by default was set to 1. * p<0.05, ** p<0.01.
Figure 4. Development time and body weight of dilp mutants.
(A) Egg-to-adult development time of dilp mutants. Only the hatching period of the adult flies is shown. Rectangle: males (m), circle: females (f). (Note: number of heterozygous flies was halved to adjust to the number of homozygous and w control flies.) (B) Body weight of dilp mutant (red) males (m) and females (f) compared to controls (black). (n = 20 flies). (C) Body weight of female flies that lack multiple dilp genes. (n = 40 f; dilp1–4,5 n = 20 f; dilp7,1–4,5 n = 18 f). _Wolbachia_+ flies (wDah) weigh more than _Wolbachia_- flies (wDahT). Body weight in (B,C): wDahT background, except for dilp1–4 mutants: w1118. ** p<0.01, t-test.
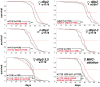
Figure 5. dilp2 mutant flies are long-lived.
Survival curves of dilp mutant flies on standard food. Lack-of DILP2 extends median lifespan in two independent genetic backgrounds (w1118 and wDahT) and in both sexes. Combined knock out of DILP2 and 3 results in increased lifespan. In contrast to MNC-ablated flies, homozygous w1118; dilp2–3,5 mutants do not show increased median lifespan. However, median lifespan is slightly increased in dilp2–3,5 heterozygous flies. ** p<0.001, log-rank test.
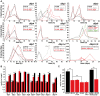
Figure 6. _Wolbachia_-dependent lifespan extension of dilp2–3,5 mutants is correlated with xenobiotic resistance.
Wolbachia affects longevity (A) and xenobiotic resistance (E) of dilp2–3,5 mutants, but not fecundity (B), starvation (C) or oxidative stress resistance (D). (A) Median and maximum lifespan of wDah;dilp2–3,5 females (83/92,5 days) compared to wDah (64,5/76 days) control females is increased by 29% and 22%, respectively. ** p<0.001, log rank test. In contrast, wDahT;dilp2–3,5 (62,5/81 days) only show a small increase in maximum, but not in median lifespan compared to wDahT (64,5/74 days) females. (B) Index of lifetime fecundity of dilp2–3,5 females is reduced to 30% but not affected by Wolbachia infection. Shown is the cumulative number of eggs laid by an average female. ** p<0.01, Wilcoxon rank sum test. (C–E) Survival of dilp2–3,5 females with and without Wolbachia on (C) 1,5% agarose, (D) 5% hydrogen peroxide and (E) DDT (275 mg/l). ** p<0.01, log rank test. (F) PCR analysis confirms the presence of Wolbachia in wDah flies and the absence in Tetracycline-treated wDahT flies. (G) Q-RT-PCR analysis shows that 4E-BP expression is not affected by _Wolbachia_-infection status. 4E-BP transcript level in _Wolbachia_- flies was normalised to the corresponding _Wolbachia_+ sample, which by default was set to 1.
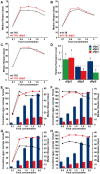
Figure 7. Dietary restriction in Drosophila is mediated by DILPs.
(A–C) dilp 2, 3 or 5 single mutants exhibit a normal response to DR compared to wild type controls. (D) Compensatory regulation among MNC-expressed DILPs on a high yeast diet. Expression levels of DILP2, 3 and 5 were measured by Q-RT-PCR on RNA extracted from heads of 10 day old dilp mutant females kept on 2.0x food. * p<0.05. (E–H) In two independent trials dilp2–3,5 mutants failed to show a normal response to DR. (E,F) wDahT; dilp2–3,5 mutants (Wolbachia-). (G,H) wDah; dilp2–3,5 mutants (Wolbachia+) Bars: index of lifetime fecundity±standard error of mean; connected points: median lifespan in days. dilp2–3,5 mutants in red, wDahT controls in black/blue.
Similar articles
- Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs.
Broughton S, Alic N, Slack C, Bass T, Ikeya T, Vinti G, Tommasi AM, Driege Y, Hafen E, Partridge L. Broughton S, et al. PLoS One. 2008;3(11):e3721. doi: 10.1371/journal.pone.0003721. Epub 2008 Nov 13. PLoS One. 2008. PMID: 19005568 Free PMC article. - Functional implications of Drosophila insulin-like peptides in metabolism, aging, and dietary restriction.
Kannan K, Fridell YW. Kannan K, et al. Front Physiol. 2013 Oct 16;4:288. doi: 10.3389/fphys.2013.00288. Front Physiol. 2013. PMID: 24137131 Free PMC article. Review. - Factors that regulate expression patterns of insulin-like peptides and their association with physiological and metabolic traits in Drosophila.
Semaniuk U, Strilbytska O, Malinovska K, Storey KB, Vaiserman A, Lushchak V, Lushchak O. Semaniuk U, et al. Insect Biochem Mol Biol. 2021 Aug;135:103609. doi: 10.1016/j.ibmb.2021.103609. Epub 2021 Jun 17. Insect Biochem Mol Biol. 2021. PMID: 34146686 Review. - Drosophila insulin-like peptide-6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin-like peptide-2 from the brain.
Bai H, Kang P, Tatar M. Bai H, et al. Aging Cell. 2012 Dec;11(6):978-85. doi: 10.1111/acel.12000. Epub 2012 Sep 18. Aging Cell. 2012. PMID: 22935001 Free PMC article. - Nutritional Geometric Profiles of Insulin/IGF Expression in Drosophila melanogaster.
Post S, Tatar M. Post S, et al. PLoS One. 2016 May 12;11(5):e0155628. doi: 10.1371/journal.pone.0155628. eCollection 2016. PLoS One. 2016. PMID: 27171400 Free PMC article.
Cited by
- Use of Drosophila as an evaluation method reveals imp as a candidate gene for type 2 diabetes in rat locus Niddm22.
Kawasaki K, Yamada S, Ogata K, Saito Y, Takahama A, Yamada T, Matsumoto K, Kose H. Kawasaki K, et al. J Diabetes Res. 2015;2015:758564. doi: 10.1155/2015/758564. Epub 2015 Mar 2. J Diabetes Res. 2015. PMID: 25821834 Free PMC article. - Genome-wide microRNA screening reveals that the evolutionary conserved miR-9a regulates body growth by targeting sNPFR1/NPYR.
Suh YS, Bhat S, Hong SH, Shin M, Bahk S, Cho KS, Kim SW, Lee KS, Kim YJ, Jones WD, Yu K. Suh YS, et al. Nat Commun. 2015 Jul 3;6:7693. doi: 10.1038/ncomms8693. Nat Commun. 2015. PMID: 26138755 Free PMC article. - Role of the hypothalamus in mediating protective effects of dietary restriction during aging.
Dacks PA, Moreno CL, Kim ES, Marcellino BK, Mobbs CV. Dacks PA, et al. Front Neuroendocrinol. 2013 Apr;34(2):95-106. doi: 10.1016/j.yfrne.2012.12.001. Epub 2012 Dec 20. Front Neuroendocrinol. 2013. PMID: 23262258 Free PMC article. Review. - The Insulin-Like Proteins dILPs-2/5 Determine Diapause Inducibility in Drosophila.
Schiesari L, Andreatta G, Kyriacou CP, O'Connor MB, Costa R. Schiesari L, et al. PLoS One. 2016 Sep 30;11(9):e0163680. doi: 10.1371/journal.pone.0163680. eCollection 2016. PLoS One. 2016. PMID: 27689881 Free PMC article.
References
- Edgar BA. How flies get their size: genetics meets physiology. Nat Rev Genet. 2006;7:907–916. - PubMed
- Broughton S, Partridge L. Insulin/IGF-like signalling, the central nervous system and aging. Biochem J. 2009;418:1–12. - PubMed
- Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51:1–24. - PubMed
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases