Binding of MetJ repressor to specific and nonspecific DNA and effect of S-adenosylmethionine on these interactions - PubMed (original) (raw)
Binding of MetJ repressor to specific and nonspecific DNA and effect of S-adenosylmethionine on these interactions
Anne M Augustus et al. Biochemistry. 2010.
Abstract
We have used analytical ultracentrifugation to characterize the binding of the methionine repressor protein, MetJ, to synthetic oligonucleotides containing zero to five specific recognition sites, called metboxes. For all lengths of DNA studied, MetJ binds more tightly to repeats of the consensus sequence than to naturally occurring metboxes, which exhibit a variable number of deviations from the consensus. Strong cooperative binding occurs only in the presence of two or more tandem metboxes, which facilitate protein-protein contacts between adjacent MetJ dimers, but weak affinity is detected even with DNA containing zero or one metbox. The affinity of MetJ for all of the DNA sequences studied is enhanced by the addition of SAM, the known cofactor for MetJ in the cell. This effect extends to oligos containing zero or one metbox, both of which bind two MetJ dimers. In the presence of a large excess concentration of metbox DNA, the effect of cooperativity is to favor populations of DNA oligos bound by two or more MetJ dimers rather than a stochastic redistribution of the repressor onto all available metboxes. These results illustrate the dynamic range of binding affinity and repressor assembly that MetJ can exhibit with DNA and the effect of the corepressor SAM on binding to both specific and nonspecific DNA.
Figures
Figure 1
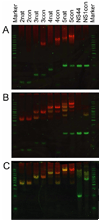
Gel shift showing MetJ-R binding to various DNA oligos in the absence (A) or presence (B) of 1 mM SAM, and in the presence of SAM and excess MetJ (C). The DNA is stained with a dye that fluoresces green while MetJ-R fluoresces red. The molecular weight marker is Hyper Ladder V from Bioline.
Figure 2
Sedimentation equilibrium study of MetJ-R alone (A) and in complex with 5con DNA (B). Residuals are in units of percent.
Figure 3
MWb values for MetJ-R complexes with DNA containing zero (NS44) or one metbox (NS1con) at various MetJ:DNA ratios. MWb for both DNAs is ~12,040. The theoretical MWb for a 1:1 MetJ:DNA complex is ~18,200 and for a 2:1 complex is ~24,400.
Figure 4
MWb values for MetJ-R complexes with DNA containing two metboxes (2con and 2nat). MWb for both DNAs is ~5440. The theoretical MWb for a 1:1 MetJ:DNA complex is ~11,600 and for a 2:1 complex is ~17,800.
Figure 5
MWb values for MetJ-R complexes with DNA containing five metboxes (5con and 5nat). MWb for both DNAs is ~12040. The theoretical MWb for a 1:1 MetJ:DNA complex is ~18,200, for a 2:1 complex is ~24,400, for a 3:1 complex is ~30,600, for a 4:1 complex is ~36,700 and for a 5:1 complex is ~42,900. The upper dashed line represents the 5:1 complex, while the lower dashed line indicates the re-equilibration curve expected for a non-cooperative process.
Figure 6
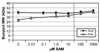
MWb values for complexes containing MetJ-R and DNA with five metboxes as a function of SAM concentration. The DNAs used were 5nat (Δ, MWb =12038), and 5con (●, MWb =12040). The DNA:MetJ was always at a 1:1 [metbox]/[MetJ-R] ratio. Stoichiometric SAM is 20 µM, indicated by the vertical dashed line between 10 and 100 µM SAM.
Figure 7
Average number (n) of MetJ-R dimers bound to stoichiometric concentrations of various DNAs in the presence (filled symbols) or absence (open symbols) of 1 mM SAM. The value of n was determined from the data using Equation 2.
Similar articles
- Structural basis for the differential regulation of DNA by the methionine repressor MetJ.
Augustus AM, Reardon PN, Heller WT, Spicer LD. Augustus AM, et al. J Biol Chem. 2006 Nov 10;281(45):34269-76. doi: 10.1074/jbc.M605763200. Epub 2006 Sep 8. J Biol Chem. 2006. PMID: 16963446 - Direct and indirect readout in mutant Met repressor-operator complexes.
Garvie CW, Phillips SE. Garvie CW, et al. Structure. 2000 Sep 15;8(9):905-14. doi: 10.1016/s0969-2126(00)00182-9. Structure. 2000. PMID: 10986458 - The Met repressor-operator complex: DNA recognition by beta-strands.
Somers WS, Rafferty JB, Phillips K, Strathdee S, He YY, McNally T, Manfield I, Navratil O, Old IG, Saint-Girons I, et al. Somers WS, et al. Ann N Y Acad Sci. 1994 Jul 29;726:105-17. doi: 10.1111/j.1749-6632.1994.tb52802.x. Ann N Y Acad Sci. 1994. PMID: 8092669 Review. - Structure and function of Escherichia coli met repressor: similarities and contrasts with trp repressor.
Phillips SE, Stockley PG. Phillips SE, et al. Philos Trans R Soc Lond B Biol Sci. 1996 Apr 29;351(1339):527-35. doi: 10.1098/rstb.1996.0051. Philos Trans R Soc Lond B Biol Sci. 1996. PMID: 8735275 Review.
Cited by
- Conversion of methionine biosynthesis in Escherichia coli from trans- to direct-sulfurylation enhances extracellular methionine levels.
Gruzdev N, Hacham Y, Haviv H, Stern I, Gabay M, Bloch I, Amir R, Gal M, Yadid I. Gruzdev N, et al. Microb Cell Fact. 2023 Aug 11;22(1):151. doi: 10.1186/s12934-023-02150-x. Microb Cell Fact. 2023. PMID: 37568230 Free PMC article. - Insights from the architecture of the bacterial transcription apparatus.
Iyer LM, Aravind L. Iyer LM, et al. J Struct Biol. 2012 Sep;179(3):299-319. doi: 10.1016/j.jsb.2011.12.013. Epub 2011 Dec 24. J Struct Biol. 2012. PMID: 22210308 Free PMC article.
References
- Gama-Castro S, Jimenez-Jacinto V, Peralta-Gil M, Santos-Zavaleta A, Penaloza-Spinola MI, Contreras-Moreira B, Segura-Salazar J, Muniz-Rascado L, Martinez-Flores I, Salgado H, Bonavides-Martinez C, Abreu-Goodger C, Rodriguez-Penagos C, Miranda-Rios J, Morett E, Merino E, Huerta AM, Trevino-Quintanilla L, Collado-Vides J. RegulonDB (version 6.0): gene regulation model of Escherichia coli K-12 beyond transcription, active (experimental) annotated promoters and Textpresso navigation. Nucleic Acids Res. 2008;36:D120–D124. - PMC - PubMed
- Old IG, Phillips SE, Stockley PG, Saint Girons I. Regulation of methionine biosynthesis in the Enterobacteriaceae. Prog Biophys Mol Biol. 1991;56:145–185. - PubMed
- Belfaiza J, Parsot C, Martel A, de la Tour CB, Margarita D, Cohen GN, Saint-Girons I. Evolution in biosynthetic pathways: two enzymes catalyzing consecutive steps in methionine biosynthesis originate from a common ancestor and possess a similar regulatory region. Proc Natl Acad Sci U S A. 1986;83:867–871. - PMC - PubMed
- Flavin M. Methionine Biosynthesis. In: Greenberg DM, editor. Metabolism of Sulphur Compounds. New York: Academic; 1975. pp. 457–503.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources