Automated electron microscopy for evaluating two-dimensional crystallization of membrane proteins - PubMed (original) (raw)
Automated electron microscopy for evaluating two-dimensional crystallization of membrane proteins
Minghui Hu et al. J Struct Biol. 2010 Jul.
Abstract
Membrane proteins fulfill many important roles in the cell and represent the target for a large number of therapeutic drugs. Although structure determination of membrane proteins has become a major priority, it has proven to be technically challenging. Electron microscopy of two-dimensional (2D) crystals has the advantage of visualizing membrane proteins in their natural lipidic environment, but has been underutilized in recent structural genomics efforts. To improve the general applicability of electron crystallography, high-throughput methods are needed for screening large numbers of conditions for 2D crystallization, thereby increasing the chances of obtaining well ordered crystals and thus achieving atomic resolution. Previous reports describe devices for growing 2D crystals on a 96-well format. The current report describes a system for automated imaging of these screens with an electron microscope. Samples are inserted with a two-part robot: a SCARA robot for loading samples into the microscope holder, and a Cartesian robot for placing the holder into the electron microscope. A standard JEOL 1230 electron microscope was used, though a new tip was designed for the holder and a toggle switch controlling the airlock was rewired to allow robot control. A computer program for controlling the robots was integrated with the Leginon program, which provides a module for automated imaging of individual samples. The resulting images are uploaded into the Sesame laboratory information management system database where they are associated with other data relevant to the crystallization screen.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
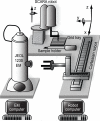
Fig. 1
Overall layout of the robotic grid loading system. The SCARA robot is responsible for loading individual EM grids from a 96-well grid tray into the EM sample holder. The Cartesian robot is responsible for picking up this sample holder and inserting it through the goniometer on the JEOL 1230 electron microscope. Axes of movement for both robots are shown. The EM computer runs Leginon and coordinates sample loading and imaging by communicating with a database server. The Robot computer queries the database and, when requested, initiates movement of the robot to insert or remove the sample.
Fig. 2
Loading EM grids. (A) A SCARA robot has been implemented for handling individual EM grids. (B) The SCARA robot is fitted with a vacuum probe to pick up grids using three nozzles positioned around the rim of the grid. (C) Grids are stored in an anodized aluminium tray with 96 wells laid out with standard SBS dimensions. Grids are transferred to this tray manually after negative staining.
Fig. 3
Modifications to the tip and the handle of the standard EM holder. (A) The holder is shown resting in a custom mount that serves to define its location and orientation relative to the SCARA and Cartesian robots. The original plastic holder was replaced with an aluminum handle, which was milled to be concentric with the rod. The bottom of the handle was also drilled with a hole that engages a pin on the mount (not seen) in order to maintain a defined position with respect to the robots. (B) A new tip with a spring-loaded clamp was designed for use with the SCARA robot. This exploded diagram shows how a spring pushes a piston against the eccentric hinge of the clamp. (C) An L-shaped finger mounted next to the vacuum probe raises the clamp. (D) The vacuum probe deposits the grid into the holder. (E) The L-shaped finger then lowers the clamp.
Fig. 4
A Cartesian robot is used to move the sample holder to the microscope and insert it through the goniometer. This robot provides three orthogonal axes for linear motion and a three-fingered gripper is mounted on a rotation stage. (A) Robot prior to picking up the sample holder. (B) Robot inserting the holder into the microscope goniometer (white arrowhead on the left).
Fig. 5
Elements of computer control connected to an Ethernet network. A Windows XP computer runs the Leginon application as well as DigitalMicrograph and communicates directly with the JEOL 1230 microscope via an RS232 serial interface. A Linux computer runs the iRobot application, which communicates with each robot via a dedicated controller. Both iRobot and Leginon communicate with second Linux computer hosting a MySQL database, which stores all the microscope calibrations and the imaging conditions as well as the images resulting from the screen.
Fig. 6
Strategy for imaging an EM grid with Leginon. As described by Cheng et al. [19], Leginon starts by recording a montage at low magnification covering a large area of the EM grid. For our application, we specified a montage containing 3x3 grid-scale images. The magnification of grid scale imaging was adjusted to include ~20 grid squares at 400 mesh. After recording the montage, Leginon evaluates individual grid squares and selects several for imaging at the square scale. We specified selection of one grid square per grid-scale image for a total of 9 per EM grid. A raster is defined at the square-scale and areas are evaluated for imaging at the hole-scale. Six examples of images at the hole scale are shown, depicting various different outcomes: 2D crystal (A), tubular crystals (B), proteoliposome (C) edge of the grid bar (D), empty grid (E) and broken carbon film (F). Scale bars correspond to 200nm in (A) and 1μm in (B-F).
Fig. 7
Incorporation of images into a Laboratory Information Management System. An image viewer has been added to the Sesame LIMS and images from screens have been imported into its database. This allows for integration of all information relevant to the crystallization screen, e.g., target protein sequence, conditions for expression and purification, crystallization trials, and crystallization score.
Similar articles
- An automated pipeline to screen membrane protein 2D crystallization.
Kim C, Vink M, Hu M, Love J, Stokes DL, Ubarretxena-Belandia I. Kim C, et al. J Struct Funct Genomics. 2010 Jun;11(2):155-66. doi: 10.1007/s10969-010-9088-5. Epub 2010 Mar 27. J Struct Funct Genomics. 2010. PMID: 20349145 Free PMC article. - A high-throughput strategy to screen 2D crystallization trials of membrane proteins.
Vink M, Derr K, Love J, Stokes DL, Ubarretxena-Belandia I. Vink M, et al. J Struct Biol. 2007 Dec;160(3):295-304. doi: 10.1016/j.jsb.2007.09.003. Epub 2007 Sep 14. J Struct Biol. 2007. PMID: 17951070 Free PMC article. - High-throughput methods for electron crystallography.
Stokes DL, Ubarretxena-Belandia I, Gonen T, Engel A. Stokes DL, et al. Methods Mol Biol. 2013;955:273-96. doi: 10.1007/978-1-62703-176-9_15. Methods Mol Biol. 2013. PMID: 23132066 Free PMC article. - Present and future of membrane protein structure determination by electron crystallography.
Ubarretxena-Belandia I, Stokes DL. Ubarretxena-Belandia I, et al. Adv Protein Chem Struct Biol. 2010;81:33-60. doi: 10.1016/B978-0-12-381357-2.00002-5. Adv Protein Chem Struct Biol. 2010. PMID: 21115172 Free PMC article. Review. - Membrane protein structure determination by electron crystallography.
Ubarretxena-Belandia I, Stokes DL. Ubarretxena-Belandia I, et al. Curr Opin Struct Biol. 2012 Aug;22(4):520-8. doi: 10.1016/j.sbi.2012.04.003. Epub 2012 May 8. Curr Opin Struct Biol. 2012. PMID: 22572457 Free PMC article. Review.
Cited by
- Inward-facing conformation of the zinc transporter YiiP revealed by cryoelectron microscopy.
Coudray N, Valvo S, Hu M, Lasala R, Kim C, Vink M, Zhou M, Provasi D, Filizola M, Tao J, Fang J, Penczek PA, Ubarretxena-Belandia I, Stokes DL. Coudray N, et al. Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):2140-5. doi: 10.1073/pnas.1215455110. Epub 2013 Jan 22. Proc Natl Acad Sci U S A. 2013. PMID: 23341604 Free PMC article. - Specimen preparation for electron diffraction of thin crystals.
Wang H, Downing KH. Wang H, et al. Micron. 2011 Feb;42(2):132-40. doi: 10.1016/j.micron.2010.05.003. Epub 2010 May 19. Micron. 2011. PMID: 20561794 Free PMC article. Review. - Structure of the SLC4 transporter Bor1p in an inward-facing conformation.
Coudray N, L Seyler S, Lasala R, Zhang Z, Clark KM, Dumont ME, Rohou A, Beckstein O, Stokes DL. Coudray N, et al. Protein Sci. 2017 Jan;26(1):130-145. doi: 10.1002/pro.3061. Epub 2016 Oct 21. Protein Sci. 2017. PMID: 27717063 Free PMC article. - Advances in structural and functional analysis of membrane proteins by electron crystallography.
Wisedchaisri G, Reichow SL, Gonen T. Wisedchaisri G, et al. Structure. 2011 Oct 12;19(10):1381-93. doi: 10.1016/j.str.2011.09.001. Structure. 2011. PMID: 22000511 Free PMC article. Review. - Leginon: New features and applications.
Cheng A, Negro C, Bruhn JF, Rice WJ, Dallakyan S, Eng ET, Waterman DG, Potter CS, Carragher B. Cheng A, et al. Protein Sci. 2021 Jan;30(1):136-150. doi: 10.1002/pro.3967. Epub 2020 Nov 3. Protein Sci. 2021. PMID: 33030237 Free PMC article.
References
- Sugahara M, Asada Y, Shimizu K, Yamamoto H, Lokanath NK, Mizutani H, Bagautdinov B, Matsuura Y, Taketa M, Kageyama Y, Ono N, Morikawa Y, Tanaka Y, Shimada H, Nakamoto T, Yamamoto M, Kunishima N. High-throughput crystallization-to-structure pipeline at RIKEN SPring-8 Center. J Struct Funct Genomics. 2008;9:21–8. - PubMed
- Wiener MC. A pedestrian guide to membrane protein crystallization. Methods. 2004;34:364–72. - PubMed
- Amos LA, Henderson R, Unwin PNT. Three-dimensional structure determination by electron microscopy of two-dimensional crystals. Prog. Biophys. Molec. Biol. 1982;39:183–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 RR17573/RR/NCRR NIH HHS/United States
- R01 GM081817/GM/NIGMS NIH HHS/United States
- R01 GM095747/GM/NIGMS NIH HHS/United States
- U54 GM094598/GM/NIGMS NIH HHS/United States
- P41 RR017573/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources