Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration - PubMed (original) (raw)
Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration
Elpida Tsika et al. J Neurosci. 2010.
Abstract
Aggregation of alpha-synuclein (alpha-syn), a process that generates oligomeric intermediates, is a common pathological feature of several neurodegenerative disorders. Despite the potential importance of the oligomeric alpha-syn intermediates in neuron function, their biochemical properties and pathobiological functions in vivo remain vastly unknown. Here we used two-dimensional analytical separation and an array of biochemical and cell-based assays to characterize alpha-syn oligomers that are present in the nervous system of A53T alpha-syn transgenic mice. The most prominent species identified were 53 A detergent-soluble oligomers, which preceded neurological symptom onset, and were found at equivalent amounts in regions containing alpha-syn inclusions as well as histologically unaffected regions. These oligomers were resistant to SDS, heat, and urea but were sensitive to proteinase-K digestion. Although the oligomers shared similar basic biochemical properties, those obtained from inclusion-bearing regions were prominently reactive to antibodies that recognize oxidized alpha-syn oligomers, significantly accelerated aggregation of alpha-syn in vitro, and caused primary cortical neuron degeneration. In contrast, oligomers obtained from non-inclusion-bearing regions were not toxic and delayed the in vitro formation of alpha-syn fibrils. These data indicate that specific conformations of alpha-syn oligomers are present in distinct brain regions of A53T alpha-syn transgenic mice. The contribution of these oligomers to the development of neuron dysfunction appears to be independent of their absolute quantities and basic biochemical properties but is dictated by the composition and conformation of the intermediates as well as unrecognized brain-region-specific intrinsic factors.
Figures
Figure 1.
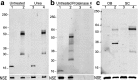
Region-specific accumulation of Triton X-100-insoluble α-syn in symptomatic A53T α-syn transgenic mice. a, Tissues from asymptomatic (4–6 months old) and symptomatic (11–12 months old) A53T α-syn transgenic mice, as well as 11-month-old transgenic mice expressing human wild-type α-syn, were subjected to extraction with 1% Triton X-100 buffer (T-soluble), followed by Western blot analysis using the human-specific anti-α-syn monoclonal antibody LB509. NSE was used as a loading control. TH served as a marker of catecholaminergic regions. Regions containing α-syn lesions [SC and cerebellum (Cr)] as well histologically unaffected regions [SN, OB, and hippocampus (H)] were analyzed. b, The insoluble pellets obtained from Triton X-100 fractions were extracted with buffer containing 2% SDS (T-insoluble). Vimentin (Vim) was used as a loading control. The markers on the left indicate the mobility of standards with known molecular masses on SDS-PAGE in kilodaltons. c, Immunohistochemical analysis demonstrating gliosis in areas of the nervous system associated with α-syn pathological inclusions. Tissue sections from olfactory bulb (A, B, E, F ) and spinal cord (C, D, G, H) from 12-month-old nTg (A, C, E, G ) and symptomatic A53T α-syn transgenic (B, D, F, H ) mice were stained with an antibody to GFAP (A–D ) or α-syn (syn505) (E–H ). Staining with syn505 reveals abundant α-syn pathological inclusions in spinal cord of the A53T α-syn transgenic mice (H) and the lack thereof in the olfactory bulb (F) and in the tissues from the nTg mice (E, G). Staining with an anti-GFAP antibody demonstrates significant gliosis in the spinal cord of the symptomatic A53T α-syn transgenic mice (D), whereas the olfactory bulbs of A53T α-syn transgenic mice (B) as well as tissues from nTg mice (A, C) demonstrate less gliosis. Scale bar, 100 μm.
Figure 2.
Identification and regional distribution of α-syn oligomers in A53T α-syn transgenic mice. Triton X-100-soluble extracts from the indicated regions of the nervous system of symptomatic mice were subjected to native SEC. The SEC fractions were then analyzed by SDS-PAGE/Western blot for α-syn using the human-specific anti-α-syn monoclonal antibody syn211. NSE was used as loading control. α-Syn monomer eluted at a volume corresponding to a 34-Å-sized particle, migrating as a 19 kDa species by SDS-PAGE. High-molecular-weight oligomers eluted between 36 and 113 Å. The horizontal marker indicates the apparent molecular radius in angstroms (Å) that corresponds to the elution volume of globular protein standards analyzed by SEC, whereas the vertical marker indicates the mobility of protein standards with known molecular mass on SDS-PAGE in kilodaltons. Representative blots of each region analyzed are shown. The graph on the bottom right shows a quantitative densitometry analysis of the SEC/SDS-PAGE blots from symptomatic and asymptomatic mice, revealing equal relative levels of soluble oligomeric α-syn from tissues of asymptomatic and symptomatic mice. A quantitative analysis of each oligomeric form is shown in supplemental Figure 2 (available at
as supplemental material). Total α-syn oligomers levels are expressed as percentage of total soluble α-syn. Mean ± SEM values; n = 3–5 for all tissues were plotted. *p = 0.02, SC symptomatic versus Hipp symptomatic, using a one-way ANOVA with Tukey's post hoc test. Combination of oligomer levels from all inclusion-bearing regions were also compared with those found in all non-inclusion-bearing regions and also did not reveal a statistically significant difference (percentage of total oligomers: regions with inclusions, 44.1 ± 9.4%, n = 9; regions without inclusions, 41.5 ± 4.6%, n = 28; mean ± SEM values; p = 0.63, Student's t test analysis). MW, Molecular weight.
Figure 3.
Biochemical analysis of the 53 Å α-syn oligomers isolated by SEC from A53T α-syn transgenic mice. The fraction corresponding to the 53-Å-sized oligomers is shown in lanes 2 and 4, and the monomer is shown in lanes 1 and 3 of each blot. a, Fractionated lysates at 0.5 mg/ml were incubated with 8
m
urea for 1 h at 25°C; b, proteinase K at 100 μg/ml for 30 min at 37°C. The results of the treatments were the same for oligomers isolated either from the SC (shown here) or OB from both symptomatic and asymptomatic mice. The human-specific α-syn monoclonal antibody LB509 (shown here) recognizes an epitope at the C terminus of the protein (residues 115-122). Analysis with the monoclonal antibody syn208 recognizing an epitope at the amyloidogenic region of the protein (residues 87-110) yielded the same results. c, Indication of biochemical diversity between oligomers isolated from regions bearing inclusions (SC) and pathology free (OB) is revealed by the monoclonal antibody syn303, which was generated against oxidized α-syn and preferentially recognizes oligomeric forms of α-syn in human brain tissue with α-syn aggregates (Duda et al., 2002).
Figure 4.
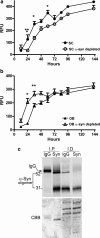
Effects of α-syn oligomers derived from symptomatic A53T α-syn transgenic mice on the kinetics of α-syn fibril formation. The kinetics of forming fibrils from recombinant purified α-syn in vitro was assessed using the amyloid binding fluorescent dye Thioflavin T and expressed as relative fluorescence units (RFU). a, Oligomers isolated from SC significantly decreased the lag phase of fibril formation of recombinant α-syn; b, oligomers isolated from the OB prolong the lag phase of fibril formation. α-Syn was depleted from oligomeric fractions by immunoprecipitation with the monoclonal antibody syn211. Values represent the mean ± SEM (n = 4–5; two-way ANOVA, *p = 0.001, **p = 0.05, ***p = 0.01). c, Western blot analysis using syn211 of immunoprecipitated (I.P.) α-syn and the corresponding immunodepleted (I.D.) fractions from the OB used in the seeding assays (similar results were obtained with the SC fractions). Total protein was visualized by Coomassie brilliant blue (CBB) staining showing equal loading of samples.
Figure 5.
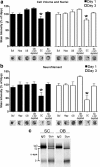
In vitro toxicity of α-syn oligomers derived from the spinal cord of symptomatic A53T α-syn transgenic mice. a, Total cell number and volume of primary mouse cortical cultures was determined by a combination of Sapphire700 (nonspecific cytosolic stain) and Draq5 (nuclear stain) after the addition of SEC fractions containing α-syn oligomers. b, Neurite degeneration was assessed by quantifying neurofilament levels by immunostaining. Twenty-five millimolar HEPES/150 m
m
NaCl, pH 7.4 (Buf), and equivalent SEC fractions from the Hipp were used as controls. SEC fractions from the SC and OB were incubated with either IgG or anti-α-syn antibodies (syn211) to deplete α-syn oligomers and determine whether neurodegeneration is mediated by the presence of α-syn (n = 5–10 for each time point; *p < 0.05). Representative immunostaining of Sapphire700/Draq5 and neurofilament from scans of the 96-well culture plate are shown under the graph. c, The amount of α-syn oligomers obtained from SC and OB SEC fractions added to the primary cultures were determined by immunoprecipitation/Western blot analysis. Samples were immunoprecipitated and blotted using the monoclonal antibody syn211 or mouse IgG as a control. The bands were analyzed by densitometry and found to be equal (integrated intensity: OB, 15.2; SC, 15.3).
Similar articles
- Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions.
Paleologou KE, Oueslati A, Shakked G, Rospigliosi CC, Kim HY, Lamberto GR, Fernandez CO, Schmid A, Chegini F, Gai WP, Chiappe D, Moniatte M, Schneider BL, Aebischer P, Eliezer D, Zweckstetter M, Masliah E, Lashuel HA. Paleologou KE, et al. J Neurosci. 2010 Mar 3;30(9):3184-98. doi: 10.1523/JNEUROSCI.5922-09.2010. J Neurosci. 2010. PMID: 20203178 Free PMC article. - Accelerated formation of alpha-synuclein oligomers by concerted action of the 20S proteasome and familial Parkinson mutations.
Lewis KA, Yaeger A, DeMartino GN, Thomas PJ. Lewis KA, et al. J Bioenerg Biomembr. 2010 Feb;42(1):85-95. doi: 10.1007/s10863-009-9258-y. Epub 2010 Feb 11. J Bioenerg Biomembr. 2010. PMID: 20148295 Free PMC article. - Neurotoxic conversion of beta-synuclein: a novel approach to generate a transgenic mouse model of synucleinopathies?
Fujita M, Sekigawa A, Sekiyama K, Sugama S, Hashimoto M. Fujita M, et al. J Neurol. 2009 Aug;256 Suppl 3:286-92. doi: 10.1007/s00415-009-5246-8. J Neurol. 2009. PMID: 19711118 Review. - Formation and development of Lewy pathology: a critical update.
Jellinger KA. Jellinger KA. J Neurol. 2009 Aug;256 Suppl 3:270-9. doi: 10.1007/s00415-009-5243-y. J Neurol. 2009. PMID: 19711116 Review.
Cited by
- Glial A30P alpha-synuclein pathology segregates neurogenesis from anxiety-related behavior in conditional transgenic mice.
Marxreiter F, Ettle B, May VE, Esmer H, Patrick C, Kragh CL, Klucken J, Winner B, Riess O, Winkler J, Masliah E, Nuber S. Marxreiter F, et al. Neurobiol Dis. 2013 Nov;59:38-51. doi: 10.1016/j.nbd.2013.07.004. Epub 2013 Jul 16. Neurobiol Dis. 2013. PMID: 23867236 Free PMC article. - Selective Neuronal Death in Neurodegenerative Diseases: The Ongoing Mystery.
Subramaniam S. Subramaniam S. Yale J Biol Med. 2019 Dec 20;92(4):695-705. eCollection 2019 Dec. Yale J Biol Med. 2019. PMID: 31866784 Free PMC article. Review. - Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson's disease.
Paumier KL, Sukoff Rizzo SJ, Berger Z, Chen Y, Gonzales C, Kaftan E, Li L, Lotarski S, Monaghan M, Shen W, Stolyar P, Vasilyev D, Zaleska M, D Hirst W, Dunlop J. Paumier KL, et al. PLoS One. 2013 Aug 1;8(8):e70274. doi: 10.1371/journal.pone.0070274. Print 2013. PLoS One. 2013. PMID: 23936403 Free PMC article. - α-Synuclein: A Multifunctional Player in Exocytosis, Endocytosis, and Vesicle Recycling.
Huang M, Wang B, Li X, Fu C, Wang C, Kang X. Huang M, et al. Front Neurosci. 2019 Jan 28;13:28. doi: 10.3389/fnins.2019.00028. eCollection 2019. Front Neurosci. 2019. PMID: 30745863 Free PMC article. Review. - Altered machinery of protein synthesis is region- and stage-dependent and is associated with α-synuclein oligomers in Parkinson's disease.
Garcia-Esparcia P, Hernández-Ortega K, Koneti A, Gil L, Delgado-Morales R, Castaño E, Carmona M, Ferrer I. Garcia-Esparcia P, et al. Acta Neuropathol Commun. 2015 Dec 1;3:76. doi: 10.1186/s40478-015-0257-4. Acta Neuropathol Commun. 2015. PMID: 26621506 Free PMC article.
References
- Apetri MM, Maiti NC, Zagorski MG, Carey PR, Anderson VE. Secondary structure of α-Synuclein oligomers: characterization by Raman and atomic force microscopy. J Mol Biol. 2006;355:63–71. - PubMed
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. - PubMed
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. - PMC - PubMed
- Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG13966/AG/NIA NIH HHS/United States
- P01 AG009215/AG/NIA NIH HHS/United States
- R01 AG013966/AG/NIA NIH HHS/United States
- AG09215/AG/NIA NIH HHS/United States
- P50 NS053488/NS/NINDS NIH HHS/United States
- R01 NS051303/NS/NINDS NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- ES013508/ES/NIEHS NIH HHS/United States
- R01NS051303/NS/NINDS NIH HHS/United States
- F32NS066730/NS/NINDS NIH HHS/United States
- F32 NS066730/NS/NINDS NIH HHS/United States
- NS053488/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous