Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains - PubMed (original) (raw)
Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains
Itaru Imayoshi et al. J Neurosci. 2010.
Abstract
Activation of Notch signaling induces the expression of transcriptional repressor genes such as Hes1, leading to repression of proneural gene expression and maintenance of neural stem/progenitor cells. However, a requirement for Notch signaling in the telencephalon was not clear, because in Hes1;Hes3;Hes5 triple-mutant mice, neural stem/progenitor cells are depleted in most regions of the developing CNS, but not in the telencephalon. Here, we investigated a role for Notch signaling in the telencephalon by generating tamoxifen-inducible conditional knock-out mice that lack Rbpj, an intracellular signal mediator of all Notch receptors. When Rbpj was deleted in the embryonic brain, almost all telencephalic neural stem/progenitor cells prematurely differentiated into neurons and were depleted. When Rbpj was deleted in the adult brain, all neural stem cells differentiated into transit-amplifying cells and neurons. As a result, neurogenesis increased transiently, but 3 months later all neural stem cells were depleted and neurogenesis was totally lost. These results indicated an absolute requirement of Notch signaling for the maintenance of neural stem cells and a proper control of neurogenesis in both embryonic and adult brains.
Figures
Figure 1.
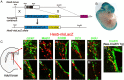
Notch signaling activity in the SVZ of the adult brain. A, Generation of Hes5-nlsLacZ knock-in mice. In the Hes5-nlsLacZ allele, the entire coding region of the Hes5 gene was replaced by the IRES-nlsLacZ expression cassette. B, X-gal staining of E12.5 Hes5-nlsLacZ embryo. C–N, Immunostaining of coronal sections of the SVZ of the lateral ventricle in Hes5-nlsLacZ mice. Most nlsLacZ+ cells expressed GFAP (D, E). A small number of nlsLacZ+ cells expressed Mash1 (F, G). However, nlsLacZ-expressing cells were negative for S100β (H, I) and DCX (J, K). Some nlsLacZ+ cells incorporated BrdU (L, M). Almost all nlsLacZ+ cells expressed CreERT2 in Hes5-nlsLacZ and Nes-CreERT2 double-transgenic mice (N).
Figure 2.
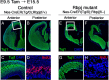
Premature loss of neural stem cells in the telencephalon of _Rbpj_-conditional knock-out embryos. A–H, Histological analysis was done with the control (A–D) and _Rbpj_-mutant (E–H) mice at E15.5. Tamoxifen was orally administered to pregnant mothers at E9.5. In control embryos, neurons (Tuj1+) reside in the outer layers and BrdU-incorporated dividing stem/progenitor cells are located in the ventricular zone (A–D). In _Rbpj_-mutant embryos, neurons are located throughout the neuroepithelium, and the number of BrdU-incorporated cells was dramatically reduced (E–H). Boxed regions in A and E are enlarged in C and G, respectively. D and H are enlarged view of the dorsal telencephalon.
Figure 3.
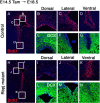
Rbpj is required for maintenance of neural stem/progenitor cells in the late embryonic telencephalon. A–N, Histological analysis was done with control (A–G) and _Rbpj_-conditional knock-out (H–N) mice at E18.5. Tamoxifen was administered to pregnant mothers at E14.5. In _Rbpj_-mutant embryos, the number of BrdU-incorporated dividing progenitors was significantly reduced in the dorsal and lateral regions of the SVZ of the lateral ventricle (H–J) compared with controls (A–C). However, in the ventral region, the number of BrdU-incorporated cells increased in _Rbpj_-mutant embryos (H, K), compared with controls (A, D). Neurons were located outside of the ventricular zone in control mice (E–G), whereas numerous DCX-positive neurons were observed in the ventricular zone of _Rbpj_-mutant mice (L–N), indicating premature neurogenesis.
Figure 4.
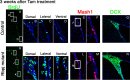
Increase in the number of transit-amplifying cells and neuroblasts in the _Rbpj_-mutant adult brain. A–N, Coronal sections of the SVZ of the lateral ventricles of control (A–G) and _Rbpj_-conditional knock-out (H–N) mice that were treated with tamoxifen 3 weeks before. BrdU was administered for 2 h before mice were killed. The number of BrdU-incorporated cells in the SVZ significantly increased in _Rbpj_-mutant mice (H–K) compared with controls (A–D). The number of Mash1+ transit-amplifying cells also increased in _Rbpj_-mutant mice (L, M) compared with controls (E, F). Boxed regions in panels E and L are enlarged in F and M, respectively. Neurogenesis (DCX+) was enhanced in _Rbpj_-mutants (N) compared with controls (G).
Figure 5.
Depletion of dividing progenitor cells and neurogenesis in the _Rbpj_-mutant adult brain. A–P, Coronal sections of the SVZ of the lateral ventricles from control (A–H) and _Rbpj_-conditional knock-out (I–P) mice that had been treated with tamoxifen 3 months before. BrdU was administered for 2 h before death. The number of cells that incorporated BrdU in the SVZ was markedly reduced in _Rbpj_-mutant mice (I–L) compared with controls (A–D). The number of Mash1+ transit-amplifying cells was also reduced in _Rbpj_-mutant mice (M, N) compared with controls (E, F). Boxed regions in panels E and M are enlarged in F and N, respectively. Neurogenesis (DCX+) was greatly attenuated in _Rbpj_-mutant mice (O, P) compared with controls (G, H).
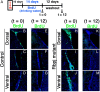
Figure 6.
Depletion of slowly dividing neural stem cells in the _Rbpj_-mutant adult brain. A, Experimental design. B–M, Dividing progenitors (t = 0; B–D, H–J) and slowly dividing cells, ones that retained labeling even after 12 d (t = 12; E–G, K–M), were labeled with BrdU in control (B–G) and _Rbpj_-mutant (H–M) mice.
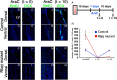
Figure 7.
Impairment of regeneration after AraC treatment in _Rbpj_-mutant adult brain. A, Experimental design. B–Q, Coronal sections of the SVZ showing BrdU-incorporating cells soon after the removal of the osmotic pump (t = 0; B–I) and 10 d after the pump removal (t = 10; J–Q) in AraC-treated control (B–E, J–M) and AraC-treated _Rbpj_-mutant (F–I, N–Q) mice. R, Quantification of the number of BrdU-incorporating cells in the SVZ. BrdU was administered for 4 h before death.
Similar articles
- Rbpj-κ mediated Notch signaling plays a critical role in development of hypothalamic Kisspeptin neurons.
Biehl MJ, Raetzman LT. Biehl MJ, et al. Dev Biol. 2015 Oct 15;406(2):235-46. doi: 10.1016/j.ydbio.2015.08.016. Epub 2015 Aug 28. Dev Biol. 2015. PMID: 26318021 Free PMC article. - Notch3 signaling promotes radial glial/progenitor character in the mammalian telencephalon.
Dang L, Yoon K, Wang M, Gaiano N. Dang L, et al. Dev Neurosci. 2006;28(1-2):58-69. doi: 10.1159/000090753. Dev Neurosci. 2006. PMID: 16508304 - Canonical Notch Signaling Directs the Fate of Differentiating Neurocompetent Progenitors in the Mammalian Olfactory Epithelium.
Herrick DB, Guo Z, Jang W, Schnittke N, Schwob JE. Herrick DB, et al. J Neurosci. 2018 May 23;38(21):5022-5037. doi: 10.1523/JNEUROSCI.0484-17.2018. Epub 2018 May 8. J Neurosci. 2018. PMID: 29739871 Free PMC article. - Regulation of neural progenitor cell development in the nervous system.
Corbin JG, Gaiano N, Juliano SL, Poluch S, Stancik E, Haydar TF. Corbin JG, et al. J Neurochem. 2008 Sep;106(6):2272-87. doi: 10.1111/j.1471-4159.2008.05522.x. J Neurochem. 2008. PMID: 18819190 Free PMC article. Review. - Notch and Neurogenesis.
Engler A, Zhang R, Taylor V. Engler A, et al. Adv Exp Med Biol. 2018;1066:223-234. doi: 10.1007/978-3-319-89512-3_11. Adv Exp Med Biol. 2018. PMID: 30030829 Review.
Cited by
- Stage-specific expression patterns and co-targeting relationships among miRNAs in the developing mouse cerebral cortex.
Todorov H, Weißbach S, Schlichtholz L, Mueller H, Hartwich D, Gerber S, Winter J. Todorov H, et al. Commun Biol. 2024 Oct 22;7(1):1366. doi: 10.1038/s42003-024-07092-7. Commun Biol. 2024. PMID: 39433948 Free PMC article. - Dendritic morphological development of traumatic brain injury-induced new neurons in the dentate gyrus is important for post-injury cognitive recovery and is regulated by Notch1.
Weston NM, Green JC, Keoprasert TN, Sun D. Weston NM, et al. Exp Neurol. 2024 Dec;382:114963. doi: 10.1016/j.expneurol.2024.114963. Epub 2024 Sep 18. Exp Neurol. 2024. PMID: 39303845 - Dynamic spatiotemporal activation of a pervasive neurogenic competence in striatal astrocytes supports continuous neurogenesis following injury.
Fogli M, Nato G, Greulich P, Pinto J, Ribodino M, Valsania G, Peretto P, Buffo A, Luzzati F. Fogli M, et al. Stem Cell Reports. 2024 Oct 8;19(10):1432-1450. doi: 10.1016/j.stemcr.2024.08.006. Epub 2024 Sep 19. Stem Cell Reports. 2024. PMID: 39303706 Free PMC article. - SOX10 mediates glioblastoma cell-state plasticity.
Man KH, Wu Y, Gao Z, Spreng AS, Keding J, Mangei J, Boskovic P, Mallm JP, Liu HK, Imbusch CD, Lichter P, Radlwimmer B. Man KH, et al. EMBO Rep. 2024 Nov;25(11):5113-5140. doi: 10.1038/s44319-024-00258-8. Epub 2024 Sep 16. EMBO Rep. 2024. PMID: 39285246 Free PMC article. - Epigenetic maintenance of adult neural stem cell quiescence in the mouse hippocampus via Setd1a.
Zhao T, Hong Y, Yan B, Huang S, Ming GL, Song H. Zhao T, et al. Nat Commun. 2024 Jul 6;15(1):5674. doi: 10.1038/s41467-024-50010-y. Nat Commun. 2024. PMID: 38971831 Free PMC article.
References
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding sonic hedgehog. Nature. 2005;437:894–897. - PubMed
- Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. - PubMed
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. - PubMed
- Baek JH, Hatakeyama J, Sakamoto S, Ohtsuka T, Kageyama R. Persistent and high levels of Hes1 expression regulate boundary formation in the developing central nervous system. Development. 2006;133:2467–2476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials