High systemic levels of interleukin-10, interleukin-22 and C-reactive protein in Indian patients are associated with low in vitro replication of HIV-1 subtype C viruses - PubMed (original) (raw)
High systemic levels of interleukin-10, interleukin-22 and C-reactive protein in Indian patients are associated with low in vitro replication of HIV-1 subtype C viruses
Juan F Arias et al. Retrovirology. 2010.
Abstract
Background: HIV-1 subtype C (HIV-1C) accounts for almost 50% of all HIV-1 infections worldwide and predominates in countries with the highest case-loads globally. Functional studies suggest that HIV-1C is unique in its biological properties, and there are contradicting reports about its replicative characteristics. The present study was conducted to evaluate whether the host cytokine environment modulates the in vitro replication capacity of HIV-1C viruses.
Methods: A small subset of HIV-1C isolates showing efficient replication in peripheral blood mononuclear cells (PBMC) is described, and the association of in vitro replication capacity with disease progression markers and the host cytokine response was evaluated. Viruses were isolated from patient samples, and the corresponding in vitro growth kinetics were determined by monitoring for p24 production. Genotype, phenotype and co-receptor usage were determined for all isolates, while clinical category, CD4 cell counts and viral loads were recorded for all patients. Plasmatic concentrations of cytokines and, acute-phase response, and microbial translocation markers were determined; and the effect of cytokine treatment on in vitro replication rates was also measured.
Results: We identified a small number of viral isolates showing high in vitro replication capacity in healthy-donor PBMC. HIV-1C usage of CXCR4 co-receptor was rare; therefore, it did not account for the differences in replication potential observed. There was also no correlation between the in vitro replication capacity of HIV-1C isolates and patients' disease status. Efficient virus growth was significantly associated with low interleukin-10 (IL-10), interleukin-22 (IL-22), and C-reactive protein (CRP) levels in plasma (p < .0001). In vitro, pretreatment of virus cultures with IL-10 and CRP resulted in a significant reduction of virus production, whereas IL-22, which lacks action on immune cells appears to mediate its anti-HIV effect through interaction with both IL-10 and CRP, and its own protective effect on mucosal membranes.
Conclusions: These results indicate that high systemic levels of IL-10, CRP and IL-22 in HIV-1C-infected Indian patients are associated with low viral replication in vitro, and that the former two have direct inhibitory effects whereas the latter acts through downstream mechanisms that remain uncertain.
Figures
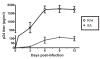
Figure 1
Viral replication kinetics of HIV-1C isolates on PHA-activated healthy-donor PBMC. Virus replication was monitored by measuring the amounts of p24 Gag protein produced in the culture supernatants every three days. The values given are mean ± SD of p24 antigen (pg/ml) of either R/H isolates (open circle) or S/L isolates (filled diamond). The data are representative of the results from three independent experiments.
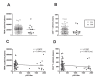
Figure 2
Viral load and CD4+ T-cell count in HIV-1C-infected Indian patients. The indicated parameters were evaluated in 85 HIV-1C-infected individuals included in this study and sorted according to viral growth phenotype. (A) Viral load (HIV RNA copies/ml plasma) in patients with either R/H (open circle) or S/L viral isolates (filled diamond). (B) CD4+ T-cell counts (cells/mm3) in the same groups of patients. (C) Lack of correlation between viral load (HIV RNA copies/ml plasma) and in vitro replication (p24, pg/ml) in the same sample. (D) Lack of correlation between CD4+ T-cell counts (cells/mm3) and in vitro replication (p24, pg/ml). For panels C and D, r = Spearman correlation coefficient; p_-value (two tailed);(ns)_ indicates that the _p_-value of the correlation coefficient was more than .05 (not significant). The mean values of the measurements obtained from two independent experiments are shown (n = 85).
Figure 3
Cytokine profile of HIV-1C infected Indian patients. The indicated cytokines, along with the inflammatory marker CRP were evaluated by ELISA in plasma collected from HIV-1C-infected patients (n = 85) harboring either R/H (filled diamond) or S/L (open circle) growth phenotype viruses, or in 10 HIV-uninfected healthy controls (filled triangle). The mean systemic values of each cytokine (pg/ml) and CRP (μg/ml) are compared in the figure for R/H and S/L viral phenotype groups. TNF-α, tumor necrosis factor-alfa; IFNγ, interferon-gamma; IL-1α, interleukin-1 α; IL-4, interleukin-4; IL-6, interleukin-6; IL-10, interleukin-10; IL-17, interleukin 17; IL-22, interleukin-22; CRP, C-reactive protein. Extreme outlier data points (IL-22) are not depicted for better visualization of results, but included into all calculations. *** = statistical difference of the medians, p < 0.001. The mean values of the measurements obtained from two independent experiments are shown.
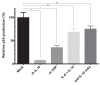
Figure 4
IL-10 and CRP pre-treatment of viral expansion cultures downregulates HIV-1C replication in vitro. PHA-activated healthy-donor PBMCs were pre-incubated with recombinant human IL-6 (2.5 ng/ml), IL-10 (5 ng/ml) or CRP (1 μg/ml), for 1 h before infection with a representative R/H phenotype HIV-1C primary isolate, and HIV-1 p24 levels were determined by ELISA after 7 days of culture. Treatments schemes are shown as follows: (1) Mock; (2) recombinant human (rh) IL-10; (3) rh CRP; (4) IL-6+IL-10; anti-IL-10 mAb+IL-10. The data are representative of the results from two independent experiments. Results are shown as mean ± SD of relative p24 antigen production expressed as percent of the mean p24 titer measured in the Mock-treated cultures. * = statistical difference of the medians, p < 0.05; ** = statistical difference of the medians, p < 0.01; (ns) indicates that the _p_-value of the correlation coefficient was more than .05 (not significant).
Figure 5
Correlation between systemic IL-22 and plasmatic CRP, LPS and IL-10 levels. (A) Systemic LPS levels were evaluated by the LAL assay, in plasma collected from HIV-1C-infected patients (n = 85) harboring either R/H (filled diamond) or S/L (open circle) growth phenotype viruses, or in 10 HIV-uninfected healthy controls (filled triangle). The mean plasmatic LPS values (pg/ml) are compared in the figure for R/H and S/L viral phenotype groups. Extreme outlier data points are not depicted for better visualization of results, but included into all calculations. *** = statistical difference of the medians, p < 0.001. (B) Correlation between plasma LPS (pg/ml) and plasma IL-22 levels (pg/ml). (C) Correlation between plasma IL-10 (pg/ml) and plasma IL-22 levels (pg/ml). (D) Correlation between plasma CRP (pg/ml) and plasma IL-22 levels (pg/ml) in our study sample (n = 85). In all panels, r = Spearman correlation coefficient; _p_-value (two tailed);_p_-values of the correlation coefficient of less than .05 were considered significant. The mean values of the measurements obtained from two independent experiments are shown.
Similar articles
- Interleukin-10-secreting CD4 cells from aged patients with AIDS decrease in-vitro HIV replication and tumour necrosis factor alpha production.
Andrade RM, Lima PG, Filho RG, Hygino J, Milczanowski SF, Andrade AF, Lauria C, Brindeiro R, Tanuri A, Bento CA. Andrade RM, et al. AIDS. 2007 Aug 20;21(13):1763-70. doi: 10.1097/QAD.0b013e3282ca83fa. AIDS. 2007. PMID: 17690575 - IL-10-secreting T cells from HIV-infected pregnant women downregulate HIV-1 replication: effect enhanced by antiretroviral treatment.
Bento CA, Hygino J, Andrade RM, Saramago CS, Silva RG, Silva AA, Linhares UC, Brindeiro R, Tanuri A, Rosenzwajg M, Klatzmann D, Andrade AF. Bento CA, et al. AIDS. 2009 Jan 2;23(1):9-18. doi: 10.1097/QAD.0b013e328317461e. AIDS. 2009. PMID: 19050381 - Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients.
Bastard JP, Soulié C, Fellahi S, Haïm-Boukobza S, Simon A, Katlama C, Calvez V, Marcelin AG, Capeau J. Bastard JP, et al. Antivir Ther. 2012;17(5):915-9. doi: 10.3851/IMP2093. Epub 2012 Mar 21. Antivir Ther. 2012. PMID: 22436412 - Interleukin-18: a proinflammatory cytokine in HIV-1 infection.
Torre D, Pugliese A. Torre D, et al. Curr HIV Res. 2006 Oct;4(4):423-30. doi: 10.2174/157016206778559993. Curr HIV Res. 2006. PMID: 17073617 Review. - Interleukin-2 and human immunodeficiency virus infection: pathogenic mechanisms and potential for immunologic enhancement.
Kinter A, Fauci AS. Kinter A, et al. Immunol Res. 1996;15(1):1-15. doi: 10.1007/BF02918280. Immunol Res. 1996. PMID: 8739561 Review.
Cited by
- Upregulation of IL-32 Isoforms in Virologically Suppressed HIV-Infected Individuals: Potential Role in Persistent Inflammation and Transcription From Stable HIV-1 Reservoirs.
Zaidan SM, Leyre L, Bunet R, Larouche-Anctil E, Turcotte I, Sylla M, Chamberland A, Chartrand-Lefebvre C, Ancuta P, Routy JP, Baril JG, Trottier B, MacPherson P, Trottier S, Harris M, Walmsley S, Conway B, Wong A, Thomas R, Kaplan RC, Landay AL, Durand M, Chomont N, Tremblay CL, El-Far M; Canadian HIV and Aging Cohort Study. Zaidan SM, et al. J Acquir Immune Defic Syndr. 2019 Dec 15;82(5):503-513. doi: 10.1097/QAI.0000000000002185. J Acquir Immune Defic Syndr. 2019. PMID: 31714430 Free PMC article. - Dynamics of Plasmatic Levels of Pro- and Anti-Inflammatory Cytokines in HIV-Infected Individuals with M. tuberculosis Co-Infection.
Nosik M, Ryzhov K, Rymanova I, Sobkin A, Kravtchenko A, Kuimova U, Pokrovsky V, Zverev V, Svitich O. Nosik M, et al. Microorganisms. 2021 Nov 4;9(11):2291. doi: 10.3390/microorganisms9112291. Microorganisms. 2021. PMID: 34835417 Free PMC article. - Interleukin-22 suppresses the growth of A498 renal cell carcinoma cells via regulation of STAT1 pathway.
Zhang F, Shang D, Zhang Y, Tian Y. Zhang F, et al. PLoS One. 2011;6(5):e20382. doi: 10.1371/journal.pone.0020382. Epub 2011 May 23. PLoS One. 2011. PMID: 21625390 Free PMC article. - Interleukin-22 reduces lung inflammation during influenza A virus infection and protects against secondary bacterial infection.
Ivanov S, Renneson J, Fontaine J, Barthelemy A, Paget C, Fernandez EM, Blanc F, De Trez C, Van Maele L, Dumoutier L, Huerre MR, Eberl G, Si-Tahar M, Gosset P, Renauld JC, Sirard JC, Faveeuw C, Trottein F. Ivanov S, et al. J Virol. 2013 Jun;87(12):6911-24. doi: 10.1128/JVI.02943-12. Epub 2013 Apr 17. J Virol. 2013. PMID: 23596287 Free PMC article. - Decreased IL-1 β Secretion as a Potential Predictor of Tuberculosis Recurrence in Individuals Diagnosed with HIV.
Nosik M, Ryzhov K, Kudryavtseva AV, Kuimova U, Kravtchenko A, Sobkin A, Zverev V, Svitich O. Nosik M, et al. Biomedicines. 2024 Apr 25;12(5):954. doi: 10.3390/biomedicines12050954. Biomedicines. 2024. PMID: 38790916 Free PMC article.
References
- Shankarappa R, Chatterjee R, Learn GH, Neogi D, Ding M, Roy P, Ghosh A, Kingsley L, Harrison L, Mullins JI, Gupta P. Human immunodeficiency virus type 1 env sequences from Calcutta in eastern India: identification of features that distinguish subtype C sequences in India from other subtype C sequences. J Virol. 2001;75:10479–10487. doi: 10.1128/JVI.75.21.10479-10487.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous