Novel findings on the metabolic effects of the low glycaemic carbohydrate isomaltulose (Palatinose) - PubMed (original) (raw)
Clinical Trial
Novel findings on the metabolic effects of the low glycaemic carbohydrate isomaltulose (Palatinose)
Ines Holub et al. Br J Nutr. 2010 Jun.
Abstract
The slow digestible disaccharide isomaltulose (iso; Palatinose) is available as novel functional carbohydrate ingredient for manufacturing of low glycaemic foods and beverages. Although basically characterised, various information on physiological effects of iso are still lacking. Thus, the objective of the present study was to expand scientific knowledge of physiological characteristics of iso by a set of three human intervention trials. Using an ileostomy model, iso was found to be essentially absorbed, irrespective of the nature of food (beverage and solid food). Apparent digestibility of 50 g iso from two different meals was 95.5 and 98.8 %; apparent absorption was 93.6 and 96.1 %, respectively. In healthy volunteers, a single dose intake of iso resulted in lower postprandial blood glucose and insulin responses than did sucrose (suc), while showing prolonged blood glucose delivery over 3 h test. In a 4-week trial with hyperlipidaemic individuals, regular consumption of 50 g/d iso within a Western-type diet was well tolerated and did not affect blood lipids. Fasting blood glucose and insulin resistance were lower after the 4-week iso intervention compared with baseline. This would be consistent with possible beneficial metabolic effects as a consequence of the lower and prolonged glycaemic response and lower insulinaemic burden. However, there was no significant difference at 4 weeks after iso compared with suc. In conclusion, the study shows that iso is completely available from the small intestine, irrespective of food matrix, leading to a prolonged delivery of blood glucose. Regular iso consumption is well tolerated also in subjects with increased risk for vascular diseases.
Figures
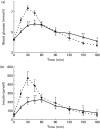
Fig. 1
Ileostomy study: apparent digestibility, absorption and excretion of isomaltulose from different meals.1, Test meal 1 was a beverage (500 ml) containing 50 g isomaltulose; 2, test meal 2 was a beverage (250 ml) and biscuits (140 g) together containing 50 g isomaltulose. ▧, Digestibility;  , absorption; ■, isomaltulose excretion.
, absorption; ■, isomaltulose excretion.
Fig. 2
Blood glucose and insulin response study. (a) Blood glucose profiles of 50 g isomaltulose ( ) and sucrose (●) over 3 h. (b) Insulin profiles of 50 g isomaltulose and sucrose over 3 h. Mean values were significantly different: *P < 0·05, **P < 0·01 by Wilcoxon test for paired data.
) and sucrose (●) over 3 h. (b) Insulin profiles of 50 g isomaltulose and sucrose over 3 h. Mean values were significantly different: *P < 0·05, **P < 0·01 by Wilcoxon test for paired data.
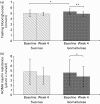
Fig. 3
Cross-over study. (a) Fasting blood glucose levels of twenty hyperlipidaemic subjects following consumption of either 50 g isomaltulose/d or 50 g sucrose/d for 4 weeks. (b) HOMA insulin resistance of twenty hyperlipidaemic subjects following consumption of either 50 g isomaltulose/d or 50 g sucrose/d for 4 weeks. Mean values were significantly different: *P < 0·05, **P < 0·01 by Wilcoxon test for paired data.
Similar articles
- Reduced glycaemic and insulinaemic responses following isomaltulose ingestion: implications for postprandial substrate use.
van Can JG, Ijzerman TH, van Loon LJ, Brouns F, Blaak EE. van Can JG, et al. Br J Nutr. 2009 Nov;102(10):1408-13. doi: 10.1017/S0007114509990687. Epub 2009 Aug 11. Br J Nutr. 2009. PMID: 19671200 Clinical Trial. - Postprandial substrate use in overweight subjects with the metabolic syndrome after isomaltulose (Palatinose™) ingestion.
König D, Theis S, Kozianowski G, Berg A. König D, et al. Nutrition. 2012 Jun;28(6):651-6. doi: 10.1016/j.nut.2011.09.019. Epub 2012 Jan 21. Nutrition. 2012. PMID: 22264450 Clinical Trial. - Comparison of the effects of slowly and rapidly absorbed carbohydrates on postprandial glucose metabolism in type 2 diabetes mellitus patients: a randomized trial.
Ang M, Linn T. Ang M, et al. Am J Clin Nutr. 2014 Oct;100(4):1059-68. doi: 10.3945/ajcn.113.076638. Epub 2014 Jul 16. Am J Clin Nutr. 2014. PMID: 25030779 Clinical Trial. - Isomaltulose (Palatinose): a review of biological and toxicological studies.
Lina BA, Jonker D, Kozianowski G. Lina BA, et al. Food Chem Toxicol. 2002 Oct;40(10):1375-81. doi: 10.1016/s0278-6915(02)00105-9. Food Chem Toxicol. 2002. PMID: 12387299 Review. - Impact of Isomaltulose on Glycemic Response in Diabetic and Healthy Populations: A Meta-Analysis.
Chen Z, Gu F, Wu J. Chen Z, et al. Nutrients. 2025 Jun 5;17(11):1940. doi: 10.3390/nu17111940. Nutrients. 2025. PMID: 40507211 Free PMC article.
Cited by
- Effect of Isomaltulose on Glycemic and Insulinemic Responses: A Systematic Review and Meta-analysis of Randomized Controlled Trials.
Xie J, Li J, Qin Q, Ning H, Long Z, Gao Y, Yu Y, Han Z, Wang F, Wang M. Xie J, et al. Adv Nutr. 2022 Oct 2;13(5):1901-1913. doi: 10.1093/advances/nmac057. Adv Nutr. 2022. PMID: 35595510 Free PMC article. - Rare sugars and their health effects in humans: a systematic review and narrative synthesis of the evidence from human trials.
Ahmed A, Khan TA, Dan Ramdath D, Kendall CWC, Sievenpiper JL. Ahmed A, et al. Nutr Rev. 2022 Jan 10;80(2):255-270. doi: 10.1093/nutrit/nuab012. Nutr Rev. 2022. PMID: 34339507 Free PMC article. - Effect of Oral Nutritional Supplements with Sucromalt and Isomaltulose versus Standard Formula on Glycaemic Index, Entero-Insular Axis Peptides and Subjective Appetite in Patients with Type 2 Diabetes: A Randomised Cross-Over Study.
Angarita Dávila L, Bermúdez V, Aparicio D, Céspedes V, Escobar MC, Durán-Agüero S, Cisternas S, de Assis Costa J, Rojas-Gómez D, Reyna N, López-Miranda J. Angarita Dávila L, et al. Nutrients. 2019 Jun 28;11(7):1477. doi: 10.3390/nu11071477. Nutrients. 2019. PMID: 31261732 Free PMC article. Clinical Trial. - Substrate Utilization and Cycling Performance Following Palatinose™ Ingestion: A Randomized, Double-Blind, Controlled Trial.
König D, Zdzieblik D, Holz A, Theis S, Gollhofer A. König D, et al. Nutrients. 2016 Jun 23;8(7):390. doi: 10.3390/nu8070390. Nutrients. 2016. PMID: 27347996 Free PMC article. Clinical Trial. - Impact of artificial sweeteners and rare sugars on the gut microbiome.
Lee CY, So YS, Yoo SH, Lee BH, Seo DH. Lee CY, et al. Food Sci Biotechnol. 2024 Jul 2;33(9):2047-2064. doi: 10.1007/s10068-024-01597-x. eCollection 2024 Jul. Food Sci Biotechnol. 2024. PMID: 39130663 Free PMC article. Review.
References
- Howlett J, Ashwell M. Glycemic response and health: summary of a workshop. Am J Clin Nutr . 2008;87(suppl):212S–216S. - PubMed
- Dickinson S, Brand-Miller J. Glycemic index, postprandial glycemia and cardiovascular disease. Curr Opin Lipidol . 2005;16:69–75. - PubMed
- Augustin LS, Franceschi S, Jenkins DJ. et al. Glycemic index in chronic disease: a review. Eur J Clin Nutr . 2002;56:1049–1071. - PubMed
- Livesey G, Taylor R, Hulshof T. et al. Glycemic response and health – a systematic review and meta-analysis: relations between dietary glycemic properties and health outcomes. Am J Clin Nutr . 2008;87(Suppl.):258S–268S. - PubMed
- Benton D, Nabb S. Carbohydrate, memory, and mood. Nutr Rev . 2003;61:S61–S67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical