Distinct factors control histone variant H3.3 localization at specific genomic regions - PubMed (original) (raw)
. 2010 Mar 5;140(5):678-91.
doi: 10.1016/j.cell.2010.01.003.
Laura A Banaszynski, Kyung-Min Noh, Peter W Lewis, Simon J Elsaesser, Sonja Stadler, Scott Dewell, Martin Law, Xingyi Guo, Xuan Li, Duancheng Wen, Ariane Chapgier, Russell C DeKelver, Jeffrey C Miller, Ya-Li Lee, Elizabeth A Boydston, Michael C Holmes, Philip D Gregory, John M Greally, Shahin Rafii, Chingwen Yang, Peter J Scambler, David Garrick, Richard J Gibbons, Douglas R Higgs, Ileana M Cristea, Fyodor D Urnov, Deyou Zheng, C David Allis
Affiliations
- PMID: 20211137
- PMCID: PMC2885838
- DOI: 10.1016/j.cell.2010.01.003
Distinct factors control histone variant H3.3 localization at specific genomic regions
Aaron D Goldberg et al. Cell. 2010.
Abstract
The incorporation of histone H3 variants has been implicated in the epigenetic memory of cellular state. Using genome editing with zinc-finger nucleases to tag endogenous H3.3, we report genome-wide profiles of H3 variants in mammalian embryonic stem cells and neuronal precursor cells. Genome-wide patterns of H3.3 are dependent on amino acid sequence and change with cellular differentiation at developmentally regulated loci. The H3.3 chaperone Hira is required for H3.3 enrichment at active and repressed genes. Strikingly, Hira is not essential for localization of H3.3 at telomeres and many transcription factor binding sites. Immunoaffinity purification and mass spectrometry reveal that the proteins Atrx and Daxx associate with H3.3 in a Hira-independent manner. Atrx is required for Hira-independent localization of H3.3 at telomeres and for the repression of telomeric RNA. Our data demonstrate that multiple and distinct factors are responsible for H3.3 localization at specific genomic locations in mammalian cells.
(c) 2010 Elsevier Inc. All rights reserved.
Figures
Figure 1. Genomic localization of histone H3.3 is dependent on amino acid sequence
A) ChIP-seq profiles across 120 MB of chromosome 1 in mouse ES cells, using antibodies as indicated. Y-axis represents the number of reads spanning a genomic position (scale on right, (baseline, maximum)). CpG islands (green) and genes (refseq (+) and refseq (−)) are shown below plots. Data for general H3 in ES cells is from (Mikkelsen et al., 2007). B) Annotations of H3 variant enriched regions. Y-axis is the number of enriched regions identified for each H3 variant, while the X-axis represents different categories of gene annotation and ES TFBS (defined in Chen et al., 2008). C) Association of H3.3 with repetitive elements. Data are presented as fold enrichment over input. SAT = satellite repeats, (TTAGGG)n = telomeric DNA repeats. See Figure S1 for details of ZFN-mediated generation and characterization of ES cell lines, and Table S1 for genome-wide correlation of ChIP-seq datasets.
Figure 2. Specific patterns of H3.3 and phosphorylated RNA polymerase at active and repressed genes
A–F) Profiles of H3 variants, H3, H3 modifications, or RNAPII as indicated above each panel across the TSS and TES for highly active (red), medium expressing (black), or low expressing (green) CpG rich genes in ES cells. Y-axis represents the average number of tags per gene per 200 bp per 1 million mapped reads. H3 data is from (Mikkelsen et al., 2007). A–E is from crosslinking ChIP-seq, while F is from native ChIP-seq. G) H3.3 is enriched around the TSS, but not into the gene body of the H3K4me3/H3K27me3 bivalent and transcriptionally repressed Zfpm2 gene in ES cells. In G-I, hybrid indicates F1 hybrid ES background. H) H3.3 and RNAPII Ser5p are enriched in the gene body and after the TES of the highly expressed gene Actb (beta-actin) in ES cells. Data for the RNAPII unphos 8WG16 track are from (Mikkelsen et al., 2007). RNA-seq data are from (Cloonan et al., 2008). I) H3.3 is enriched around the TSS and into the body of the non-coding RNA gene Gas5, but less enriched in the gene body of the neighboring low expressing Zbtb37 gene in ES cells. Data is represented as in Figure 1D. Gene is shown to scale below plot, with start and direction or transcription indicated by arrow. Green rectangles above the gene represent CpG islands. See also Figure S2.
Figure 3. Cell-type specific enrichment of H3.3 at transcription factor binding sites and developmentally regulated genes
A) H3.3, H3K4me1, and H3K36me3 are enriched in the gene body of the highly expressed pluripotency gene Esrrb in ES cells, and this enrichment is largely lost upon differentiation to NPCs. B) The epidermal growth factor receptor gene Egfr is H3K4me3/H3K27me3 bivalent and transcriptionally repressed in ES cells. Upon differentiation to NPCs, H3.3, H3K4me1, and H3K36me3 are enriched in the Egfr gene body. C) H3.3, H3K4me1, and H3K36me3 are enriched in the gene body of the housekeeping gene lactate dehydrogenase A Ldha in both ES cells and NPCs, and is not enriched in the neighboring Ldhc gene. The Y-axes in panels A-C are identical (indicated in the right side of panel C). D–E) H3.3 is enriched genome-wide around ES cell TFBS. Crosslinking ChIP-seq genome-wide profiles of H3 variants as indicated around ES binding sites for Nanog, Oct4, Sox2, Smad1, and STAT3. TFBS in mouse ES cells were from a previous ChIP-seq study (Chen et al., 2008), and classified as promoter (black), gene body (dark blue), or intergenic (light blue). Panel D represents data from ES cells, while panel E represents data from NPCs at identical regions. Y-axis represents number of tags per binding site per 200 bp per 1 million mapped reads. Data for H3 in ES cells is from (Mikkelsen et al., 2007). In A–E, “(native)” indicates native H3.3-HA ChIP-seq, and “(replicate)” indicates biological replicate H3.3-HA ChIP-seq from F1 hybrid ES background. F) H3.3 and H3K4me1 are enriched at an intergenic region bound by multiple transcription factors (Chen et al., 2008) specifically in ES cells. G) H3.3 and H3K4me1 are enriched at an intergenic region specifically in NPCs. See also Figure S3.
Figure 4. Enrichment of H3.3 at active and repressed genes is Hira-dependent
A) Immunoblots showing expression of H3.3-EYFP in wild-type and Hira−/− ES cells, with antibodies as indicated. B–E) Native ChIP-seq profiles of H3 variants, in wild-type ES cells (B-D) and Hira −/− ES cells (E) across the TSS and TES for highly active (red), medium expressing (black), or low expressing (green) CpG rich genes, with data represented as in Figure 2. See also Figure S4. F) H3.3 enrichment at the TSS of repressed bivalent gene Pparg is Hira-dependent. G) H3.3 enrichment at the active, ribosomal-protein coding gene Rps19 is Hira-dependent.
Figure 5. Hira is not essential for H3.3 enrichment at transcription factor binding sites
A) Native ChIP-seq genome-wide profiles of H3.3 around previously described ES TFBS (Chen et al., 2008), with data analyzed as in Figure 3. B–C) Localization of H3.3 in ES cells at intergenic enhancer elements near Mmp17 (B) and Foxi3 (C) is Hira-independent. See also Figure S5 and Table S3.
Figure 6. Atrx and Daxx association with H3.3 is specific, Hira-independent, and conserved in mouse and human cells
A) H3.3-EYFP, H3.2-EYFP, H3.1-EYFP, and Hira −/− H3.3-EYFP associated proteins were immunopurified from heterozygous H3.3B ES cells via the EYFP tag, resolved by SDS-PAGE, and visualized by Coomassie Blue. Proteins were identified by mass spectrometry, and those listed in red are specific to H3.3. The arrow indicating Hira points to a polypeptide in the H3.3 lane running immediately above the polypeptide Nasp (*), which is common to all purified H3 variants. A full list of identified proteins is presented in Table S4. B) Immunoblots of immunopurified H3 variant associated proteins from ES cells with antibodies as indicated. See also Figure S6. C) Immunoblots of Atrx, Daxx and FLAG-HA-H3 from HeLa cells stably expressing H3.3-FLAG-HA (FHA) or H3.1-FHA (Tagami et al., 2004). D) Oligonucleosomes purified from H3.3-FHA and H3.1-FHA HeLa cells. Micrococcal nuclease digested chromatin was incubated with M2 agarose beads in order to purify H3.3-FHA and H3.1-FHA oligonucleosomes and associated proteins. Two adjacent FLAG elution fractions (FE1 and FE2) from each H3.3-FHA and H3.1-FHA purification are shown in D–E. Oligonucleosomal DNA was purified and run on a 1% agarose gel, with fragment size indicated. E) Immunoblots of eluate from two adjacent FLAG elution fractions (FE1 and FE2) following FLAG affinity purification from H3.3-FHA and H3.1-FHA oligonucleosomes (D).
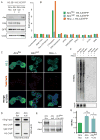
Figure 7. Atrx is required for Hira-independent enrichment of H3.3 at telomeres, and for repression of telomeric repeat-containing RNA
A) Immunoblots showing expression of H3.3-EYFP in Atrxflox, Atrxnull, and Hira −/− ES cells, with antibodies as indicated. B) ChIP-seq analysis of H3.3 enrichment at repetitive elements in Atrxflox, Atrxnull, and Hira −/− ES cells. Fold enrichment of repetitive elements in ChIP-seq data are plotted over input. C) Immunofluorescence (IF) and telomere fluorescence in situ hybridization (FISH) of Atrxflox, Atrxnull, and Hira −/− ES cells expressing H3.3B/H3.3-EYFP. Representative confocal images of interphase ES cells demonstrate that H3.3 co-localization with telomeres is lost in Atrxnull ES cells (middle), but maintained in Hira −/− ES cells (right). Bar = 5μm. D) ChIP of Atrx, control IgG, and H3 was performed in Atrxflox and Atrxnull ES cells, followed by slot blot with a telomere probe. E) Immunoblots of Atrx in Atrxflox, Atrxnull, and Atrxflox ES cells 4 days after treatment with retroviral Cre. Atrxt refers to a previously identified alternative splicing product of Atrx that remains expressed in Atrxnull ES cells (Garrick et al., 2006). F) Representative northern blot for TERRA (upper panel) and Gapdh (lower panel) in Atrxflox and Atrxnull ES cells in the presence and absence of Cre. To ensure that signal is from RNA, samples on the right were treated with RNase for 30 minutes as indicated. G) Quantification of TERRA normalized to Gapdh. Error bars represent the standard deviation of three independent experiments. See also Figure S7.
Similar articles
- H3.Y discriminates between HIRA and DAXX chaperone complexes and reveals unexpected insights into human DAXX-H3.3-H4 binding and deposition requirements.
Zink LM, Delbarre E, Eberl HC, Keilhauer EC, Bönisch C, Pünzeler S, Bartkuhn M, Collas P, Mann M, Hake SB. Zink LM, et al. Nucleic Acids Res. 2017 Jun 2;45(10):5691-5706. doi: 10.1093/nar/gkx131. Nucleic Acids Res. 2017. PMID: 28334823 Free PMC article. - The histone H3.3 chaperone HIRA restrains erythroid-biased differentiation of adult hematopoietic stem cells.
Murdaugh RL, Hoegenauer KA, Kitano A, Holt MV, Hill MC, Shi X, Tiessen JF, Chapple R, Hu T, Tseng YJ, Lin A, Martin JF, Young NL, Nakada D. Murdaugh RL, et al. Stem Cell Reports. 2021 Aug 10;16(8):2014-2028. doi: 10.1016/j.stemcr.2021.06.009. Epub 2021 Jul 8. Stem Cell Reports. 2021. PMID: 34242617 Free PMC article. - HIRA protects telomeres against R-loop-induced instability in ALT cancer cells.
Lynskey ML, Brown EE, Bhargava R, Wondisford AR, Ouriou JB, Freund O, Bowman RW 2nd, Smith BA, Lardo SM, Schamus-Hayes S, Hainer SJ, O'Sullivan RJ. Lynskey ML, et al. Cell Rep. 2024 Nov 26;43(11):114964. doi: 10.1016/j.celrep.2024.114964. Epub 2024 Nov 6. Cell Rep. 2024. PMID: 39509271 Free PMC article. - A Molecular Prospective for HIRA Complex Assembly and H3.3-Specific Histone Chaperone Function.
Ricketts MD, Marmorstein R. Ricketts MD, et al. J Mol Biol. 2017 Jun 30;429(13):1924-1933. doi: 10.1016/j.jmb.2016.11.010. Epub 2016 Nov 19. J Mol Biol. 2017. PMID: 27871933 Free PMC article. Review. - HIRA vs. DAXX: the two axes shaping the histone H3.3 landscape.
Choi J, Kim T, Cho EJ. Choi J, et al. Exp Mol Med. 2024 Feb;56(2):251-263. doi: 10.1038/s12276-023-01145-3. Epub 2024 Feb 1. Exp Mol Med. 2024. PMID: 38297159 Free PMC article. Review.
Cited by
- One identity or more for telomeres?
Giraud-Panis MJ, Pisano S, Benarroch-Popivker D, Pei B, Le Du MH, Gilson E. Giraud-Panis MJ, et al. Front Oncol. 2013 Mar 15;3:48. doi: 10.3389/fonc.2013.00048. eCollection 2013. Front Oncol. 2013. PMID: 23509004 Free PMC article. - Histone deposition pathways determine the chromatin landscapes of H3.1 and H3.3 K27M oncohistones.
Sarthy JF, Meers MP, Janssens DH, Henikoff JG, Feldman H, Paddison PJ, Lockwood CM, Vitanza NA, Olson JM, Ahmad K, Henikoff S. Sarthy JF, et al. Elife. 2020 Sep 9;9:e61090. doi: 10.7554/eLife.61090. Elife. 2020. PMID: 32902381 Free PMC article. - Exploring the Molecular Underpinnings of Cancer-Causing Oncohistone Mutants Using Yeast as a Model.
Zhang X, Fawwal DV, Spangle JM, Corbett AH, Jones CY. Zhang X, et al. J Fungi (Basel). 2023 Dec 11;9(12):1187. doi: 10.3390/jof9121187. J Fungi (Basel). 2023. PMID: 38132788 Free PMC article. Review. - Tumour immune landscape of paediatric high-grade gliomas.
Ross JL, Velazquez Vega J, Plant A, MacDonald TJ, Becher OJ, Hambardzumyan D. Ross JL, et al. Brain. 2021 Oct 22;144(9):2594-2609. doi: 10.1093/brain/awab155. Brain. 2021. PMID: 33856022 Free PMC article. Review. - Histone chaperone ASF1B promotes human β-cell proliferation via recruitment of histone H3.3.
Paul PK, Rabaglia ME, Wang CY, Stapleton DS, Leng N, Kendziorski C, Lewis PW, Keller MP, Attie AD. Paul PK, et al. Cell Cycle. 2016 Dec;15(23):3191-3202. doi: 10.1080/15384101.2016.1241914. Epub 2016 Oct 18. Cell Cycle. 2016. PMID: 27753532 Free PMC article.
References
- Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell. 2002;9:1191–1200. - PubMed
- Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. - PubMed
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. - PubMed
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. - PubMed
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125:315–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DP1 DA026192/DA/NIDA NIH HHS/United States
- DP1DA026192/DA/NIDA NIH HHS/United States
- BB/E009603/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_U137961147/MRC_/Medical Research Council/United Kingdom
- RR022220/RR/NCRR NIH HHS/United States
- P41 RR000862/RR/NCRR NIH HHS/United States
- U54 RR022220/RR/NCRR NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R37 GM053512/GM/NIGMS NIH HHS/United States
- GM07739/GM/NIGMS NIH HHS/United States
- BHF_/British Heart Foundation/United Kingdom
- GM53512/GM/NIGMS NIH HHS/United States
- RR00862/RR/NCRR NIH HHS/United States
- GM53122/GM/NIGMS NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases