Evaluation of an algorithm to guide patients with type 1 diabetes treated with continuous subcutaneous insulin infusion on how to respond to real-time continuous glucose levels: a randomized controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.2337/dc09-1481. Epub 2010 Mar 9.
Balasubramanium Krishnamurthy, James D Best, Fergus J Cameron, Peter G Colman, Steven Farish, Peter S Hamblin, Michele A O'Connell, Christine Rodda, Kevin Rowley, Helena Teede, David N O'Neal
Affiliations
- PMID: 20215457
- PMCID: PMC2875432
- DOI: 10.2337/dc09-1481
Randomized Controlled Trial
Evaluation of an algorithm to guide patients with type 1 diabetes treated with continuous subcutaneous insulin infusion on how to respond to real-time continuous glucose levels: a randomized controlled trial
Alicia J Jenkins et al. Diabetes Care. 2010 Jun.
Erratum in
- Diabetes Care. 2010 Aug;33(8):1911
Abstract
Objective: To evaluate an algorithm guiding responses of continuous subcutaneous insulin infusion (CSII)-treated type 1 diabetic patients using real-time continuous glucose monitoring (RT-CGM).
Research design and methods: Sixty CSII-treated type 1 diabetic participants (aged 13-70 years, including adult and adolescent subgroups, with A1C <or=9.5%) were randomized in age-, sex-, and A1C-matched pairs. Phase 1 was an open 16-week multicenter randomized controlled trial. Group A was treated with CSII/RT-CGM with the algorithm, and group B was treated with CSII/RT-CGM without the algorithm. The primary outcome was the difference in time in target (4-10 mmol/l) glucose range on 6-day masked CGM. Secondary outcomes were differences in A1C, low (<or=3.9 mmol/l) glucose CGM time, and glycemic variability. Phase 2 was the week 16-32 follow-up. Group A was returned to usual care, and group B was provided with the algorithm. Glycemia parameters were as above. Comparisons were made between baseline and 16 weeks and 32 weeks.
Results: In phase 1, after withdrawals 29 of 30 subjects were left in group A and 28 of 30 subjects were left in group B. The change in target glucose time did not differ between groups. A1C fell (mean 7.9% [95% CI 7.7-8.2to 7.6% [7.2-8.0]; P < 0.03) in group A but not in group B (7.8% [7.5-8.1] to 7.7 [7.3-8.0]; NS) with no difference between groups. More subjects in group A achieved A1C <or=7% than those in group B (2 of 29 to 14 of 29 vs. 4 of 28 to 7 of 28; P = 0.015). In phase 2, one participant was lost from each group. In group A, A1C returned to baseline with RT-CGM discontinuation but did not change in group B, who continued RT-CGM with addition of the algorithm.
Conclusions: Early but not late algorithm provision to type 1 diabetic patients using CSII/RT-CGM did not increase the target glucose time but increased achievement of A1C <or=7%. Upon RT-CGM cessation, A1C returned to baseline.
Figures
Figure 1
Flow of participants through the study.
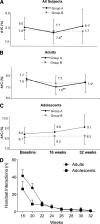
Figure 2
A1C at baseline, 16 weeks, and 32 weeks according to study group in all participants (A), adult participants (B), and adolescent participants (C) who returned for the 32-week visit. D: Number of interactions with handsets providing “reactive” guidelines on how to respond to real-time glucose levels. *P < 0.05, compared with baseline for the 16-week data and compared with 16 weeks for the 32-week data. **P < 0.0001, 16 weeks compared with baseline.
Similar articles
- An algorithm guiding patient responses to real-time-continuous glucose monitoring improves quality of life.
Jenkins AJ, Krishnamurthy B, Best JD, Cameron FJ, Colman PG, Hamblin PS, O'Connell MA, Rodda C, Teede H, O'Neal DN. Jenkins AJ, et al. Diabetes Technol Ther. 2011 Feb;13(2):105-9. doi: 10.1089/dia.2010.0139. Diabetes Technol Ther. 2011. PMID: 21284476 Clinical Trial. - Real-time continuous glucose monitoring or continuous subcutaneous insulin infusion, what goes first?: results of a pilot study.
Moreno-Fernandez J, Gomez FJ, Gazquez M, Pedroche M, García-Manzanares A, Tenias JM, Benito P, Gomez IR. Moreno-Fernandez J, et al. Diabetes Technol Ther. 2013 Jul;15(7):596-600. doi: 10.1089/dia.2013.0033. Epub 2013 Apr 30. Diabetes Technol Ther. 2013. PMID: 23631604 Clinical Trial. - Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study.
Raccah D, Sulmont V, Reznik Y, Guerci B, Renard E, Hanaire H, Jeandidier N, Nicolino M. Raccah D, et al. Diabetes Care. 2009 Dec;32(12):2245-50. doi: 10.2337/dc09-0750. Epub 2009 Sep 18. Diabetes Care. 2009. PMID: 19767384 Free PMC article. Clinical Trial. - Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis.
Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, Wilson LM, Haberl EB, Brick J, Bass EB, Golden SH. Yeh HC, et al. Ann Intern Med. 2012 Sep 4;157(5):336-47. doi: 10.7326/0003-4819-157-5-201209040-00508. Ann Intern Med. 2012. PMID: 22777524 Review. - Integrated sensor-augmented pump therapy systems [the MiniMed® Paradigm™ Veo system and the Vibe™ and G4® PLATINUM CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation.
Riemsma R, Corro Ramos I, Birnie R, Büyükkaramikli N, Armstrong N, Ryder S, Duffy S, Worthy G, Al M, Severens J, Kleijnen J. Riemsma R, et al. Health Technol Assess. 2016 Feb;20(17):v-xxxi, 1-251. doi: 10.3310/hta20170. Health Technol Assess. 2016. PMID: 26933827 Free PMC article. Review.
Cited by
- HbA1c variability in adults with type 1 diabetes on continuous subcutaneous insulin infusion (CSII) therapy compared to multiple daily injection (MDI) treatment.
Scott ES, McGrath RT, Januszewski AS, Calandro D, Hardikar AA, O'Neal DN, Fulcher G, Jenkins AJ. Scott ES, et al. BMJ Open. 2019 Dec 29;9(12):e033059. doi: 10.1136/bmjopen-2019-033059. BMJ Open. 2019. PMID: 31888933 Free PMC article. - Cutaneous Complications With Continuous or Flash Glucose Monitoring Use: Systematic Review of Trials and Observational Studies.
Asarani NAM, Reynolds AN, Boucher SE, de Bock M, Wheeler BJ. Asarani NAM, et al. J Diabetes Sci Technol. 2020 Mar;14(2):328-337. doi: 10.1177/1932296819870849. Epub 2019 Aug 27. J Diabetes Sci Technol. 2020. PMID: 31452386 Free PMC article. - Future Perspectives in Glucose Monitoring Sensors.
Frontino G, Meschi F, Bonfanti R, Rigamonti A, Battaglino R, Favalli V, Bonura C, Ferro G, Chiumello G. Frontino G, et al. Eur Endocrinol. 2013 Mar;9(1):6-11. doi: 10.17925/EE.2013.09.01.21. Epub 2013 Mar 15. Eur Endocrinol. 2013. PMID: 30349603 Free PMC article. Review. - Clinical Efficacy of Two Different Methods to Initiate Sensor-Augmented Insulin Pumps: A Randomized Controlled Trial.
Moreno-Fernandez J, Gómez FJ, Gálvez Moreno MÁ, Castaño JP. Moreno-Fernandez J, et al. J Diabetes Res. 2016;2016:4171789. doi: 10.1155/2016/4171789. Epub 2016 Nov 29. J Diabetes Res. 2016. PMID: 28004007 Free PMC article. Clinical Trial. - Influences on Technology Use and Efficacy in Type 1 Diabetes.
Franklin V. Franklin V. J Diabetes Sci Technol. 2016 May 3;10(3):647-55. doi: 10.1177/1932296816639315. Print 2016 May. J Diabetes Sci Technol. 2016. PMID: 27022096 Free PMC article. Review.
References
- Mastrototaro JJ, Cooper KW, Soundararajan G, Sanders JB, Shah RV: Clinical experience with an integrated continuous glucose sensor/insulin pump platform: a feasibility study. Adv Ther 2006;23:725–732 - PubMed
- Mastrototaro JJ, Soundararajan G, Cooper K, Shah R: Accuracy of real time continuous glucose monitoring in the Minimed Paradigm system (Abstract): Diabetes 2007;56(Suppl. 1):0422-P
- Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF: Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970;19:644–655 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous