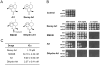Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation - PubMed (original) (raw)
Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation
Juan Wang et al. PLoS One. 2010.
Abstract
The biological mode of action of artemisinin, a potent antimalarial, has long been controversial. Previously we established a yeast model addressing its mechanism of action and found mitochondria the key in executing artemisinin's action. Here we present data showing that artemisinin directly acts on mitochondria and it inhibits malaria in a similar way as yeast. Specifically, artemisinin and its homologues exhibit correlated activities against malaria and yeast, with the peroxide bridge playing a key role for their inhibitory action in both organisms. In addition, we showed that artemisinins are distributed to malarial mitochondria and directly impair their functions when isolated mitochondria were tested. In efforts to explore how the action specificity of artemisinin is achieved, we found strikingly rapid and dramatic reactive oxygen species (ROS) production is induced with artemisinin in isolated yeast and malarial but not mammalian mitochondria, and ROS scavengers can ameliorate the effects of artemisinin. Deoxyartemisinin, which lacks an endoperoxide bridge, has no effect on membrane potential or ROS production in malarial mitochondria. OZ209, a distantly related antimalarial endoperoxide, also causes ROS production and depolarization in isolated malarial mitochondria. Finally, interference of mitochondrial electron transport chain (ETC) can alter the sensitivity of the parasite towards artemisinin. Addition of iron chelator desferrioxamine drastically reduces ETC activity as well as mitigates artemisinin-induced ROS production. Taken together, our results indicate that mitochondrion is an important direct target, if not the sole one, in the antimalarial action of artemisinins. We suggest that fundamental differences among mitochondria from different species delineate the action specificity of this class of drugs, and differing from many other drugs, the action specificity of artemisinins originates from their activation mechanism.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
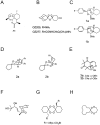
Figure 1. Artemisinin and its antimalarial derivatives include various peroxide compounds.
(A) Artemisinin. (B) OZ209 and OZ277. (C–E) Enantiomers (1a, 1b; 2a, 2b and 3a, 3b) that have been synthesized and shown with no stereoselective difference in antimalarial activity. (F) Yingzhaosu A. (G and H) Antimalarial tetraoxanes.
Figure 2. The peroxidic bridge is essential to the inhibitory activity of artemisinin in both yeast and malaria parasites.
(A) Molecular structures of artemisinin and analogues (artemisinins) used in the study. Deoxyartemisinin contains only one O in the otherwise O-O bridge. (B) Inhibition of yeast growth with artemisinins. Strains of _ndi1_Δ and _nde1_Δ, with mutated internal and external NADH dehydrogenase (type II complex I) respectively, exhibit reduced sensitivity to artemisinins. (C) Inhibition of P. falciparum growth by artemisinins in cell culture. Data were shown as mean ± SEM.
Figure 3. Artemisinin acts by depolarizing mitochondrial membrane of malaria parasites and yeast cells.
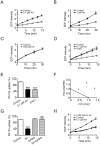
(A,B) Artemisinin treatment resulted in loss of Δψm (mitochondrial membrane potential) in P. berghei (A) and yeast (B). The membrane potential could be mostly recovered by washing off artemisinin shortly after treatment. Δψm was monitored by the fluorescence intensity of DiOC6(3) or rhodamine 123 (Rh123). Higher fluorescence intensity of stained cells indicates higher Δψm. n = 5. (C) Effect of artemisinin and deoxyartemisinin on the membrane potential of isolated malarial mitochondria. Isolated P. berghei mitochondria were incubated with artemisinin or deoxyartemisinin. Loss of membrane potential was apparent with 100 nM artemisinin treatment but not visible with 8 µM deoxyartemisinin. Δψm was assessed by measuring the Δψm-dependent uptake of Rh123. n = 3. Art, artemisinin. Deoxy-Art, deoxyartemisinin (D) Effect of artemisinin and deoxyartemisinin on membrane potential of isolated yeast mitochondria. Isolated yeast mitochondria were incubated with 1 µM artemisinin or 8 µM deoxyartemisinin. Δψm was assessed by measuring the Δψm-dependent uptake of Rh123. n = 3. Art, artemisinin. Deoxy-Art, deoxyartemisinin (E) Artemisinin selectively depolarizes malarial mitochondria but not CHO mitochondria. Mitochondria isolated from P. berghei and CHO cells were mixed and treated with 100 nM artemisinin. Artemisinin is not effective on the mammalian mitochondria. n = 3. Data were shown as mean ± SEM. * p<0.05 versus control; ** p<0.01 versus control.
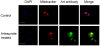
Figure 4. Artemisinins are distributed to the mitochondria.
Immunofluorescence analysis revealed the subcellular localization of artesunate. Artesunate-treated P. berghei cells were stained with DAPI, Mitotracker Red and monoclonal antibody against artesuate. The top and bottom panels are cells without or with artesuate treatment, respectively. Some of the artesuate immunofluorescence signal is colocalized with mitochondria. Scale bar, 5 µm.
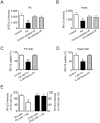
Figure 5. Artemisinin results in ROS production in the malaria mitochondria but not in mammalian mitochondria.
(A–C) Artemisinin promotes ROS generation in isolated mitochondria from malaria parasites (A) and yeast (B) but not in mammalian mitochondria (C). ROS production was monitored by measuring DCF fluorescence intensity. (D,E) Comparable amount of ROS generated by X/XO system as artemisinin resulted in similar degree of uncoupling effect on isolated malarial mitochondria. Δψm of isolated P. berghei mitochondria was assessed by measuring the Δψm-dependent uptake of Rh123. ROS production was monitored using dichlorofluorescin diacetate (DCFH-DA) assay. (F) An isobologram for DPI and artemisinin. Data points above the line indicate antagonism between ROS scavenger DPPD and artemisinin. FIC: fractional inhibitory concentration. (G) ROS scavenger DPPD and its effect on the depolarizing activity of artemisnin. (H) Deoxyartemisinin has no effect on ROS production in isolated malaria mitochondria. n = 3. Data were shown as mean ± SEM. *** p<0.0001 versus control, ### p<0.0001 versus Art.
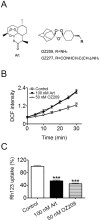
Figure 6. OZ209, a synthetic endoperoxide antimalarial with structurally different backbone, also affects the malarial mitochondrial functions.
(A) Molecular structures of artemisinin, OZ209 and OZ277. (B) OZ209 and its effect on the ROS production in isolated malarial mitochondria. ROS production in isolated P. berghei mitochondria was monitored by measuring DCF fluorescence intensity. (C) OZ209 and its depolarizing effect on mitochondrial membrane. Δψ of isolated P. berghei mitochondria was assessed by measuring the Δψ-dependent uptake of Rh123. n = 3. Data were shown as mean ± SEM. *** p<0.0001 versus control.
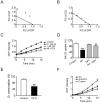
Figure 7. Interference of the electron transport chain affects the artemisinin's action.
(A) An isobologram for DPI and artemisinin. Data points above the line indicate antagonism between DPI and artemisinin. FIC: fractional inhibitory concentration. DPI: diphenylene iodonium chloride. (B) An isobologram for DPI and chloroquine (CQ). (C) Effect of DPI on the ROS promoting ability of artemisinin. ROS was monitored using the DCFH-DA assay. (D) Effect of DPI on the depolarizing ability of artemisinin. Δψ of isolated P. berghei mitochondria was assessed by measuring the Δψ-dependent uptake of Rh123. (E) Iron chelator DFO inhibits ETC activity. (F) Iron chelator DFO reduces artemisinin-induced ROS production in isolated malarial mitochondria. n = 3. Data were shown as mean ± SEM. ** p<0.01 versus conrol; *** p<0.001 versus control; ## p<0.01 versus Art.
Similar articles
- Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.
Haynes RK, Cheu KW, N'Da D, Coghi P, Monti D. Haynes RK, et al. Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review. - Yeast model uncovers dual roles of mitochondria in action of artemisinin.
Li W, Mo W, Shen D, Sun L, Wang J, Lu S, Gitschier JM, Zhou B. Li W, et al. PLoS Genet. 2005 Sep;1(3):e36. doi: 10.1371/journal.pgen.0010036. PLoS Genet. 2005. PMID: 16170412 Free PMC article. - Artemisinin Derivatives and Synthetic Trioxane Trigger Apoptotic Cell Death in Asexual Stages of Plasmodium.
Gunjan S, Sharma T, Yadav K, Chauhan BS, Singh SK, Siddiqi MI, Tripathi R. Gunjan S, et al. Front Cell Infect Microbiol. 2018 Jul 26;8:256. doi: 10.3389/fcimb.2018.00256. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30094226 Free PMC article. - A mitochondria-targeting artemisinin derivative with sharply increased antitumor but depressed anti-yeast and anti-malaria activities.
Sun C, Cao Y, Zhu P, Zhou B. Sun C, et al. Sci Rep. 2017 Apr 3;7:45665. doi: 10.1038/srep45665. Sci Rep. 2017. PMID: 28368011 Free PMC article. - Exploration of artemisinin derivatives and synthetic peroxides in antimalarial drug discovery research.
Patel OPS, Beteck RM, Legoabe LJ. Patel OPS, et al. Eur J Med Chem. 2021 Mar 5;213:113193. doi: 10.1016/j.ejmech.2021.113193. Epub 2021 Jan 18. Eur J Med Chem. 2021. PMID: 33508479 Review.
Cited by
- Sesquiterpenoids lactones: benefits to plants and people.
Chadwick M, Trewin H, Gawthrop F, Wagstaff C. Chadwick M, et al. Int J Mol Sci. 2013 Jun 19;14(6):12780-805. doi: 10.3390/ijms140612780. Int J Mol Sci. 2013. PMID: 23783276 Free PMC article. Review. - Artemisinin protects against cerebral ischemia and reperfusion injury via inhibiting the NF-κB pathway.
Ji H, Jin H, Li G, Jin L, Ren X, Lv Y, Wang Y. Ji H, et al. Open Med (Wars). 2022 May 11;17(1):871-881. doi: 10.1515/med-2022-0435. eCollection 2022. Open Med (Wars). 2022. PMID: 35950034 Free PMC article. - Remodeling of the malaria parasite and host human red cell by vesicle amplification that induces artemisinin resistance.
Bhattacharjee S, Coppens I, Mbengue A, Suresh N, Ghorbal M, Slouka Z, Safeukui I, Tang HY, Speicher DW, Stahelin RV, Mohandas N, Haldar K. Bhattacharjee S, et al. Blood. 2018 Mar 15;131(11):1234-1247. doi: 10.1182/blood-2017-11-814665. Epub 2018 Jan 23. Blood. 2018. PMID: 29363540 Free PMC article. - A barcode of organellar genome polymorphisms identifies the geographic origin of Plasmodium falciparum strains.
Preston MD, Campino S, Assefa SA, Echeverry DF, Ocholla H, Amambua-Ngwa A, Stewart LB, Conway DJ, Borrmann S, Michon P, Zongo I, Ouédraogo JB, Djimde AA, Doumbo OK, Nosten F, Pain A, Bousema T, Drakeley CJ, Fairhurst RM, Sutherland CJ, Roper C, Clark TG. Preston MD, et al. Nat Commun. 2014 Jun 13;5:4052. doi: 10.1038/ncomms5052. Nat Commun. 2014. PMID: 24923250 Free PMC article. - H2O2 dynamics in the malaria parasite Plasmodium falciparum.
Rahbari M, Rahlfs S, Jortzik E, Bogeski I, Becker K. Rahbari M, et al. PLoS One. 2017 Apr 3;12(4):e0174837. doi: 10.1371/journal.pone.0174837. eCollection 2017. PLoS One. 2017. PMID: 28369083 Free PMC article.
References
- Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. - PubMed
- White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. - PubMed
- O'Neill PM, Posner GH. A medicinal chemistry perspective on artemisinin and related endoperoxides. J Med Chem. 2004;47:2945–2964. - PubMed
- China Cooperative Research Group on Qinghaosu and Its Derivatives as Antimalarials. Chemical studies on qinghaosu (artemisinine). J Trad Chin Med. 1982;2:3–8. - PubMed
- Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE. Current perspectives on the mechanism of action of artemisinins. Int J Parasitol. 2006;36:1427–1441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases