VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts - PubMed (original) (raw)
. 2010 Apr 21;29(8):1377-88.
doi: 10.1038/emboj.2010.30. Epub 2010 Mar 11.
Fuad Bahram, Xiujuan Li, Laura Gualandi, Sina Koch, Malin Jarvius, Ola Söderberg, Andrey Anisimov, Ivana Kholová, Bronislaw Pytowski, Megan Baldwin, Seppo Ylä-Herttuala, Kari Alitalo, Johan Kreuger, Lena Claesson-Welsh
Affiliations
- PMID: 20224550
- PMCID: PMC2868571
- DOI: 10.1038/emboj.2010.30
VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts
Ingrid Nilsson et al. EMBO J. 2010.
Abstract
The vascular endothelial growth factors VEGFA and VEGFC are crucial regulators of vascular development. They exert their effects by dimerization and activation of the cognate receptors VEGFR2 and VEGFR3. Here, we have used in situ proximity ligation to detect receptor complexes in intact endothelial cells. We show that both VEGFA and VEGFC potently induce formation of VEGFR2/-3 heterodimers. Receptor heterodimers were found in both developing blood vessels and immature lymphatic structures in embryoid bodies. We present evidence that heterodimers frequently localize to tip cell filopodia. Interestingly, in the presence of VEGFC, heterodimers were enriched in the leading tip cells as compared with trailing stalk cells of growing sprouts. Neutralization of VEGFR3 to prevent heterodimer formation in response to VEGFA decreased the extent of angiogenic sprouting. We conclude that VEGFR2/-3 heterodimers on angiogenic sprouts induced by VEGFA or VEGFC may serve to positively regulate angiogenic sprouting.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
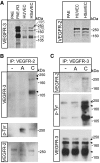
Induction of VEGFR2/-3 heterodimers in co-expressing endothelial cells. (A) Total cell lysates derived from HUVECs, HSaVECS or PAE cells; either untransfected or expressing VEGFR3 were immunoblotted to detect VEGFR3 (left panel). After reduction of disulphide bridges, VEGFR3 migrates as three species of apparent mw 195, 175 and 125 kDa. PAE, HUVEC and HSaVEC lysates were also immunoblotted to detect VEGFR2, which migrates as two species around the 250 kDa marker (right panel). (B) HSaVEC lysates from cells treated or not with VEGFA or VEGFC for 8 min were used for immunoprecipitation (ip) of VEGFR2 followed by immunoblotting for VEGFR3 (upper panel) to detect receptor heterodimerization. This was followed by immunoblotting to detect phosphorylated VEGFR2 (middle panel) using the 4G10 mAb, and immunoblotting to show equal loading of VEGFR2 (lower panel). (C) Immunoprecipitation of VEGFR3 from HSaVECs treated as in (B) followed by immunoblotting to detect co-immunoprecipitation of VEGFR2 (upper panel), phosphorylation of VEGFR3 (middle panel) and VEGFR3 loading (lower panel).
Figure 2
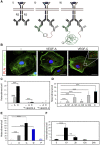
In situ PLA detection of VEGFR2/-3 heterodimers in intact HSaVECs. (A) Schematic outline of the in situ PLA strategy showing: (i) dimerized receptors (VEGFR2 in blue and VEGFR3 in grey) reacting with primary antibodies; (ii) close proximity of oligonucleotide-ligated secondary antibodies allows a rolling-circle amplification (RCA); (iii) detection of the RCA product by a fluorescently labelled probe. (B) Detection of heterodimers (in red) in HSaVECs treated with vehicle (–), VEGFA or VEGFC for 8 min on cells labelled with FITC-conjugated phalloidin (green). Inset in the VEGFC panel shows high magnification to clearly visualize the PLA spots representing heterodimers. Scale bar=10 μm. (C) Quantification of VEGFR2/-3 heterodimers in HSaVECs treated with vehicle (–), VEGFA (A) or VEGFC (C) in cells preincubated or not with neutralizing antibodies blocking ligand binding to VEGFR2 or VEGFR3. _n_=6. (D) Quantification of VEGFR2/-3 heterodimers in HSaVECs treated with different human VEGF isoforms (VEGFA121, 145, 165 or 189) or VEGFC for 8 min. _n_=6. (E) Quantification of VEGFR2/-3 heterodimers in response to VEGFA, VEGFC, VEGFD or PDGFB. Growth factors are indicated as A (VEGFA), C (VEGFC), D (VEGFD) and P (PDGFB). _n_=6. Note that a different batch of PLA probes was used in this analysis compared with other panels in the figure (see Materials and methods). (F) Turnover of VEGFR2/-3 heterodimers in HSaVECs. Cells were treated with VEGFC for different time periods from 10 min to 24 h and samples were processed for detection of in situ PLA signals. _n_=6. Asterisks in panels C–F indicate the degree of significance (**P<0.01, ***P<0.001).
Figure 3
VEGFR2/-3 homo- and heterodimers induced by VEGFA or VEGFC. (A) Schematic outline of primary antibody ligation with oligonucleotide plus and minus strands to detect VEGFR homodimers. Only pairing of antibodies with plus and minus strands allow initiation of the rolling-circle amplification. (B) VEGFR2/-3 heterodimerization. HSaVECs were treated for 8 min with either VEGFA or VEGFC. Heterodimerization was 3–4-fold more efficiently induced by VEGFC. _n_=6. (C) VEGFR2 homodimers. Equal mixtures of VEGFR2 monoclonal antibodies ligated with plus and minus strands of oligonucleotides (as outlined in A) were used to detect VEGFR2 homodimers on HSaVECs treated for 8 min with VEGFA or VEGFC as above. _n_=6. (D) VEGFR3 homodimers. Equal mixtures of VEGFR3 monoclonal antibodies ligated with plus and minus strands of oligonucleotides were used to detect VEGFR3 homodimers on HSaVECs treated for 8 min with VEGFA or VEGFC. _n_=6. (E) Schematic outline of VEGFR homo- and heterodimerization induced by VEGFA or VEGFC. Note that the relative distribution of homodimers versus heterodimers cannot be accurately determined due to the inherent difference in affinity of different antibodies. Asterisks in panels B–D indicate the degree of significance (***P<0.001).
Figure 4
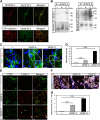
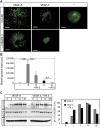
Formation of vessel structures in differentiating 2D EBs involves both VEGFR2 and VEGFR3. (A) Expression of VEGFRs in 2D EB vascular structures. EBs differentiating in 2D cultures for 12 days in the presence of VEGFA shows vessel-like structures co-expressing VEGFR2 and VEGFR3 (upper panels; merged immunostainings to the right), or VE-cadherin and VEGFR3 (lower panels; merged immunostainings to the right). Scale bar=50 μm (upper), 100 μm (lower). (B) Complex formation between VEGFRs in 2D EBs. Left: Immunoprecipitation (ip) of VEGFR3 from day 12 EBs treated with vehicle (–) VEGFA (A) or VEGFC (C) for 15 min followed by immunoblotting for VEGFR2 (upper left panel). Immunoblotting for VEGFR3 (lower left panel) shows equal loading of VEGFR3. Right: Parallel aliquots of cell lysate were analysed by immunoprecipitation of VEGFR2 followed by immunoblotting for VEGFR3 (upper right panel). VEGFR2 immunoblotting (lower right panel) showed equal loading. (C) Heterodimers in CD31-positive cells. Formation of VEGFR2/-3 heterodimers as detected by in situ PLA (red spots) on 2D EBs immunostained for CD31 (green), in response to vehicle (–), VEGFA or VEGFC. Scale bar=10 μm. (D) Quantification of PLA spots in CD31-positive cells as in C. _n_=8. (E) Identification of LYVE1-positive cells. EBs in 2D cultures were treated with VEGFA or VEGFC until day 12, and immunostained to detect expression of CD31 (green) and LYVE1 (red). Panels to the right show merged immunostainings. Lymphatic vascular structures expressing LYVE1 but not CD31 are indicated by arrows in the VEGFC-treated cultures. Single, rather than vessel-organized LYVE1-positive cells, are indicated by asterisk. Scale bar=100 μm. (F) Heterodimers in LYVE1-positive cells. EBs in 2D culture were treated as indicated above and processed for immunostaining to detect CD31 (blue) and LYVE1 (white), followed by in situ PLA to detect VEGFR2/-3 heterodimers (red). Scale bar=10 μm. (G) Quantification of PLA spots in LYVE1-positive cells as in (F). _n_=8. Asterisks in panels D and G indicate the degree of significance (**P<0.01, ***P<0.001).
Figure 5
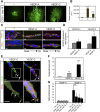
Heterodimers in angiogenic sprouts. (A) Angiogenic sprouting in response to VEGFA or VEGFC. EBs were cultured in 3D collagen matrix in the presence of VEGFA or VEGFC. Microphotographs were taken at day 18 on whole-mount fixed, CD31-immunostained samples. Scale bar=300 μm. (B) Quantification of total vascular area from the data in (A) based on five EBs per condition and expressed as fold induction ±s.d. (C) Expression of VEGFR2 and VEGFR3 in angiogenic sprouts. Immunostaining for VEGFR2 (green; upper panels) and VEGFR3 (green; lower panels) on CD31-positive (red) angiogenic sprouts in 3D EBs treated with VEGFA or VEGFC. The orientation of the tip versus stalk is indicated. Scale bar=10 μm. (D) Quantification of VEGFR2 and VEGFR3 expression in the tip cell region compared with entire angiogenic sprouts. _n_=4. (E) Location of heterodimers on VEGFC-induced angiogenic sprouts. Red spots represent PLA reactions in tip cells. Panels show PLA spots in CD31-positive angiogenic tip cell regions. Lower panels show saturation of CD31-positivity to better visualize the filopodia. Note that PLA spots are located on filopodia extending ahead of the tip cell. Scale bar=10 μm. (F) Quantification of in situ PLA detecting VEGFR2/-3 heterodimers in angiogenic sprouts in response to VEGFA or VEGFC in 3D EB cultures. _n_=8. (G) Quantification of heterodimers in tip and stalk cells. VEGFA-treated angiogenic sprouts contained heterodimers evenly distributed over the sprouts, whereas VEGFC-treated angiogenic sprouts showed accumulation of heterodimers in tip cells. _n_=8. Asterisks in panels B, D, F–G indicate degree of significance (*P<0.05, **P<0.01, ***P<0.001).
Figure 6
VEGFA-induced angiogenic sprouting is mediated by VEGFR2 homodimers and VEGFR2/-3 heterodimers. (A) Effect of VEGFR3 neutralization. VEGFA- or VEGFC-induced EB cultures in 3D collagen were treated with or without antibodies neutralizing the function of VEGFR3. Scale bar=300 μm. (B) Quantification of sprouting area. Cultures treated with VEGFA or VEGFC in the absence or presence of neutralizing VEGFR3 antibodies as shown in panel (A) were quantified. _n_=5. Asterisks indicate degree of significance (**P<0.01). (C) Activation of VEGFR2. Immunoblotting for phosphorylated VEGFR2 (pY1175) from HSaVEC cultures treated with VEGFA in the presence and absence of neutralizing VEGFR3 antibodies, for different time periods (left panel). Middle panel shows blotting for VEGFR2 and lower panel shows equal loading of β-actin. Densitometric scanning (right panel) of bands showed no decrease in VEGFR2 activation by the VEGFR3 antibodies.
Similar articles
- Molecular controls of lymphatic VEGFR3 signaling.
Deng Y, Zhang X, Simons M. Deng Y, et al. Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):421-9. doi: 10.1161/ATVBAHA.114.304881. Epub 2014 Dec 18. Arterioscler Thromb Vasc Biol. 2015. PMID: 25524775 Free PMC article. - VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling.
Heinolainen K, Karaman S, D'Amico G, Tammela T, Sormunen R, Eklund L, Alitalo K, Zarkada G. Heinolainen K, et al. Circ Res. 2017 Apr 28;120(9):1414-1425. doi: 10.1161/CIRCRESAHA.116.310477. Epub 2017 Mar 15. Circ Res. 2017. PMID: 28298294 Free PMC article. - Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling.
Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Benedito R, et al. Nature. 2012 Mar 18;484(7392):110-4. doi: 10.1038/nature10908. Nature. 2012. PMID: 22426001 - VEGFRs and Notch: a dynamic collaboration in vascular patterning.
Jakobsson L, Bentley K, Gerhardt H. Jakobsson L, et al. Biochem Soc Trans. 2009 Dec;37(Pt 6):1233-6. doi: 10.1042/BST0371233. Biochem Soc Trans. 2009. PMID: 19909253 Review. - Dynamics of endothelial cell behavior in sprouting angiogenesis.
Eilken HM, Adams RH. Eilken HM, et al. Curr Opin Cell Biol. 2010 Oct;22(5):617-25. doi: 10.1016/j.ceb.2010.08.010. Curr Opin Cell Biol. 2010. PMID: 20817428 Review.
Cited by
- Actin cytoskeleton in angiogenesis.
Yadunandanan Nair N, Samuel V, Ramesh L, Marib A, David DT, Sundararaman A. Yadunandanan Nair N, et al. Biol Open. 2022 Dec 15;11(12):bio058899. doi: 10.1242/bio.058899. Epub 2022 Nov 29. Biol Open. 2022. PMID: 36444960 Free PMC article. Review. - uPARAP/Endo180 receptor is a gatekeeper of VEGFR-2/VEGFR-3 heterodimerisation during pathological lymphangiogenesis.
Durré T, Morfoisse F, Erpicum C, Ebroin M, Blacher S, García-Caballero M, Deroanne C, Louis T, Balsat C, Van de Velde M, Kaijalainen S, Kridelka F, Engelholm L, Struman I, Alitalo K, Behrendt N, Paupert J, Noel A. Durré T, et al. Nat Commun. 2018 Dec 5;9(1):5178. doi: 10.1038/s41467-018-07514-1. Nat Commun. 2018. PMID: 30518756 Free PMC article. - Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation.
Leppänen VM, Tvorogov D, Kisko K, Prota AE, Jeltsch M, Anisimov A, Markovic-Mueller S, Stuttfeld E, Goldie KN, Ballmer-Hofer K, Alitalo K. Leppänen VM, et al. Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):12960-5. doi: 10.1073/pnas.1301415110. Epub 2013 Jul 22. Proc Natl Acad Sci U S A. 2013. PMID: 23878260 Free PMC article. - Spatial regulation of receptor tyrosine kinases in development and cancer.
Casaletto JB, McClatchey AI. Casaletto JB, et al. Nat Rev Cancer. 2012 May 24;12(6):387-400. doi: 10.1038/nrc3277. Nat Rev Cancer. 2012. PMID: 22622641 Free PMC article. Review. - AIP1 mediates vascular endothelial cell growth factor receptor-3-dependent angiogenic and lymphangiogenic responses.
Zhou HJ, Chen X, Huang Q, Liu R, Zhang H, Wang Y, Jin Y, Liang X, Lu L, Xu Z, Min W. Zhou HJ, et al. Arterioscler Thromb Vasc Biol. 2014 Mar;34(3):603-15. doi: 10.1161/ATVBAHA.113.303053. Epub 2014 Jan 9. Arterioscler Thromb Vasc Biol. 2014. PMID: 24407031 Free PMC article.
References
- Adams RH, Alitalo K (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 8: 464–478 - PubMed
- Alitalo K, Tammela T, Petrova TV (2005) Lymphangiogenesis in development and human disease. Nature 438: 946–953 - PubMed
- Baldwin ME, Catimel B, Nice EC, Roufail S, Hall NE, Stenvers KL, Karkkainen MJ, Alitalo K, Stacker SA, Achen MG (2001) The specificity of receptor binding by vascular endothelial growth factor-d is different in mouse and man. J Biol Chem 276: 19166–19171 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous