Adenosine protected against pulmonary edema through transporter- and receptor A2-mediated endothelial barrier enhancement - PubMed (original) (raw)
Adenosine protected against pulmonary edema through transporter- and receptor A2-mediated endothelial barrier enhancement
Qing Lu et al. Am J Physiol Lung Cell Mol Physiol. 2010 Jun.
Abstract
We have previously demonstrated that adenosine plus homocysteine enhanced endothelial basal barrier function and protected against agonist-induced barrier dysfunction in vitro through attenuation of RhoA activation by inhibition of isoprenylcysteine-O-carboxyl methyltransferase. In the current study, we tested the effect of elevated adenosine on pulmonary endothelial barrier function in vitro and in vivo. We noted that adenosine alone dose dependently enhanced endothelial barrier function. While adenosine receptor A(1) or A(3) antagonists were ineffective, an adenosine transporter inhibitor, NBTI, or a combination of DPMX and MRS1754, antagonists for adenosine receptors A(2A) and A(2B), respectively, partially attenuated the barrier-enhancing effect of adenosine. Similarly, inhibition of both A(2A) and A(2B) receptors with siRNA also blunted the effect of adenosine on barrier function. Interestingly, inhibition of both transporters and A(2A)/A(2B) receptors completely abolished adenosine-induced endothelial barrier enhancement. The adenosine receptor A(2A) and A(2B) agonist, NECA, also significantly enhanced endothelial barrier function. These data suggest that both adenosine transporters and A(2A) and A(2B) receptors are necessary for exerting maximal effect of adenosine on barrier enhancement. We also found that adenosine enhanced Rac1 GTPase activity and overexpression of dominant negative Rac1 attenuated adenosine-induced increases in focal adhesion complexes. We further demonstrated that elevation of cellular adenosine by inhibition of adenosine deaminase with Pentostatin significantly enhanced endothelial basal barrier function, an effect that was also associated with enhanced Rac1 GTPase activity and with increased focal adhesion complexes and adherens junctions. Finally, using a non-inflammatory acute lung injury (ALI) model induced by alpha-naphthylthiourea, we found that administration of Pentostatin, which elevated lung adenosine level by 10-fold, not only attenuated the development of edema before ALI but also partially reversed edema after ALI. The data suggest that adenosine deaminase inhibition may be useful in treatment of pulmonary edema in settings of ALI.
Figures
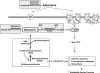
Fig. 1.
Schematic representation of adenosine uptake and metabolism pathways and extracellular adenosine receptor-mediated pathways. TP, adenosine transporters.
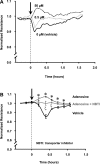
Fig. 2.
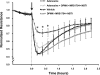
Effects of adenosine and its transporters on pulmonary artery endothelial monolayer barrier function. Pulmonary artery endothelial cells (PAEC) were incubated with indicated concentrations of adenosine (A) or incubated with vehicle (0.02% DMSO in medium) or 50 μM adenosine (in 0.02% DMSO-containing medium) in the presence or absence of 10 μM NBTI for 1 h (B). Changes in endothelial monolayer permeability were assessed by assaying changes in electrical resistance across the monolayers using the electrical cell impedance system (ECIS). A representative tracing from 6 independent experiments is presented in A. The means ± SE of the normalized resistance are presented in B, n = 6, *P < 0.05 vs. vehicle; ξ_P_ < 0.05 vs. adenosine or vehicle. Arrows indicate the time of addition of treatments.
Fig. 3.
Effects of A1 and A3 receptors on the barrier-enhancing effect of adenosine. PAEC were incubated with vehicle (0.4% DMSO in medium) or 50 μM adenosine (in 0.4% DMSO-containing medium) in the presence or absence of 50 μM DPCPX (A) or 10 μM MRS1911 (B) for 1 h. Changes in endothelial monolayer permeability were assessed by assaying changes in electrical resistance across the monolayers using ECIS. The means ± SE of the normalized resistance are presented. A: n = 3–4; B: n = 5–6, *P < 0.05 vs. vehicle. Arrows indicate the time of addition of treatments.
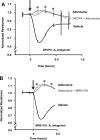
Fig. 4.
Effect of A2A and A2B receptors on the barrier-enhancing effect of adenosine. PAEC were incubated with vehicle (0.1% DMSO in medium) or 50 μM NECA for 1 h (A) or incubated with vehicle (0.5% DMSO) or 50 μM adenosine (in 0.5% DMSO-containing medium) in the presence or absence of 50 μM DPMX (B) or 10 μM MRS1754 (C) or 50 μM DPMX + 10 μM MRS1754 (D) for 1 h. E and F: PAEC were transfected with control (scrambled) siRNA or A2A siRNA + A2B siRNA for 72 h. Knockdown of A2A and A2B mRNA was assessed by real-time RT-PCR. The transfected cells were then treated with vehicle or 50 μM adenosine for 1 h. Changes in endothelial monolayer permeability were assessed by assaying changes in electrical resistance across the monolayers using ECIS. The means ± SE of the normalized resistance are presented. A: n = 4–5; B: n = 3–4; C: n = 5–6; D: n = 5–6; E is a representative tracing from 3 independent experiments; F represents the means ± SE of the normalized resistance at 15-min posttreatments. *P < 0.05 vs. vehicle; ξ_P_ < 0.05 vs. adenosine or vehicle. Arrows indicate the time of addition of treatments.
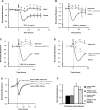
Fig. 5.
Inhibition of adenosine receptor A2 and transport diminished barrier-protective effect of adenosine. PAEC were incubated with vehicle (0.62% DMSO in medium) or 50 μM adenosine (in 0.62% DMSO-containing medium) in the presence or absence of 50 μM DPMX, 10 μM MRS1754, and 10 μM NBTI. Changes in endothelial monolayer permeability were assessed by assaying changes in electrical resistance across the monolayers using ECIS. The means ± SE of the normalized resistance are presented. *P < 0.05 compared with all other treatments. Arrows indicate the time of addition of treatments.
Fig. 6.
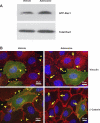
Effects of adenosine on Rac1 activity and formation of focal adhesion complexes and adherens junctions. A: PAEC were incubated with vehicle or 50 μM adenosine for 15 min. Rac1 GTPase activity was assessed by measuring the level of GTP-Rac1 by precipitation assay using GST-PBD beads. Representative immunoblots from 3 independent experiments are shown. B: PAEC were transfected with pEGFP-C1 or dominant negative Rac1 (pcDNA3-EGFP-Rac1-T17N) for 24 h. Cells were then treated with vehicle or 50 μM adenosine for 15 min and then fixed. Focal adhesion complexes and adherens junctions were immunofluorescently assessed by staining vinculin and β-catenin, respectively. The data on cells transfected with dominant negative Rac1 are shown, which represent 3 independent experiments. Green indicates GFP-Rac1-T17N overexpressing cells, and red indicates vinculin or β-catenin staining. Asterisks indicate intercellular gaps. Arrowheads indicate vinculin or β-catenin staining in vehicle-treated cells, whereas the arrows indicate vinculin or β-catenin staining in adenosine-treated cells. Scale bar is 25 μm.
Fig. 7.
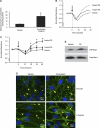
Effects of Pentostatin on intracellular adenosine level and endothelial barrier function in vitro. A: PAEC were incubated with vehicle (PBS) or 1 mg/ml Pentostatin for 1 h, and cell pellets were lysed. Adenine nucleosides were extracted from lysates, and intracellular adenosine levels were quantitated using HPLC. The data are presented as means ± SE of nanomoles per milligram of protein; n = 4. *P < 0.05 vs. vehicle. B and C: PAEC were incubated with vehicle (PBS) or indicated concentrations of Pentostatin for 1 h. Changes in endothelial monolayer permeability were assessed by ECIS. A representative tracing is presented in B. The means ± SE of the normalized resistance are presented in C; n = 8, *P < 0.05 vs. vehicle. Arrows indicate the time for addition of treatments. D: PAEC were incubated with vehicle (PBS) or 2 mg/ml Pentostatin for 30 min and then fixed. Focal adhesion complexes and adherens junctions were immunofluorescently assessed by staining vinculin and β-catenin, respectively. Representative pictures from 3 independent experiments are shown. Asterisks indicate intercellular gaps. Arrowheads indicate vinculin or β-catenin staining in vehicle-treated cells, whereas the arrows indicate vinculin or β-catenin staining in adenosine-treated cells. Scale bar is 25 μm. E: PAEC were incubated with vehicle (PBS) or 2 mg/ml Pentostatin for 30 min. Rac1 GTPase activity was assessed by measuring Rac1-GTP by precipitation assay using GST-PBD beads. Representative immunoblots from 2 independent experiments are shown.
Fig. 8.
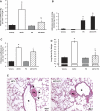
The effect of Pentostatin on α-naphthylthiourea (ANTU)-induced lung edema. A: Sprague-Dawley rats were subcutaneously administered 2 doses of saline (vehicle) or Pentostatin (40 mg/kg−1) at 0 and 15 h. The rats were then intraperitoneally given vehicle (Tween 80) or 10 mg/kg−1 ANTU at 16.5 h. Animals were then killed at 20.5 h, lungs were isolated and perfused at a constant flow, and filtration coefficients were determined. B–E: rats were intraperitoneally administered vehicle or 10 mg/kg−1 ANTU and then subcutaneously given vehicle or Pentostatin (40 mg/kg−1) at 1 h following the initial ANTU injections. Lungs were isolated at 3 h after Pentostatin injection. Lung adenosine level (B), filtration coefficient (C), lung wet-to-dry weight ratio (D), and lung histology (E) were determined. A: n = 3–7, *P < 0.001 vs. vehicle or Pentostatin, ξ_P_ < 0.05 vs. ANTU. B: n = 3, *P < 0.001 vs. vehicle or ANTU. C: n = 3–8, *P < 0.001 vs. vehicle or Pentostatin, ξ_P_ < 0.05 vs. ANTU. D: n = 3–8, *P < 0.0001 vs. vehicle or Pentostatin, ξ_P_ < 0.05 vs. ANTU. E: n = 2, representative images were obtained at ×200 magnification and are presented. Arrows indicate perivascular cuffing. Scale bar is 50 μm.
Similar articles
- Sustained adenosine exposure causes lung endothelial barrier dysfunction via nucleoside transporter-mediated signaling.
Lu Q, Newton J, Hsiao V, Shamirian P, Blackburn MR, Pedroza M. Lu Q, et al. Am J Respir Cell Mol Biol. 2012 Nov;47(5):604-13. doi: 10.1165/rcmb.2012-0012OC. Epub 2012 Jun 28. Am J Respir Cell Mol Biol. 2012. PMID: 22744860 Free PMC article. - Junctional complex and focal adhesion rearrangement mediates pulmonary endothelial barrier enhancement by FTY720 S-phosphonate.
Wang L, Bittman R, Garcia JG, Dudek SM. Wang L, et al. Microvasc Res. 2015 May;99:102-9. doi: 10.1016/j.mvr.2015.03.007. Epub 2015 Apr 7. Microvasc Res. 2015. PMID: 25862132 Free PMC article. - Isoprenylcysteine carboxyl methyltransferase modulates endothelial monolayer permeability: involvement of RhoA carboxyl methylation.
Lu Q, Harrington EO, Hai CM, Newton J, Garber M, Hirase T, Rounds S. Lu Q, et al. Circ Res. 2004 Feb 20;94(3):306-15. doi: 10.1161/01.RES.0000113923.85084.C1. Epub 2003 Dec 29. Circ Res. 2004. PMID: 14699010 - Barrier dysfunction and RhoA activation are blunted by homocysteine and adenosine in pulmonary endothelium.
Harrington EO, Newton J, Morin N, Rounds S. Harrington EO, et al. Am J Physiol Lung Cell Mol Physiol. 2004 Dec;287(6):L1091-7. doi: 10.1152/ajplung.00421.2003. Epub 2004 Jul 30. Am J Physiol Lung Cell Mol Physiol. 2004. PMID: 15286003 - Extracellular adenosine-induced Rac1 activation in pulmonary endothelium: Molecular mechanisms and barrier-protective role.
Kovacs-Kasa A, Kim KM, Cherian-Shaw M, Black SM, Fulton DJ, Verin AD. Kovacs-Kasa A, et al. J Cell Physiol. 2018 Aug;233(8):5736-5746. doi: 10.1002/jcp.26281. Epub 2018 Mar 7. J Cell Physiol. 2018. PMID: 29168172 Free PMC article.
Cited by
- Protective effect of adenosine receptors against lipopolysaccharide-induced acute lung injury.
Gonzales JN, Gorshkov B, Varn MN, Zemskova MA, Zemskov EA, Sridhar S, Lucas R, Verin AD. Gonzales JN, et al. Am J Physiol Lung Cell Mol Physiol. 2014 Mar 15;306(6):L497-507. doi: 10.1152/ajplung.00086.2013. Epub 2014 Jan 10. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24414256 Free PMC article. - Differential mechanisms of adenosine- and ATPγS-induced microvascular endothelial barrier strengthening.
Bátori R, Kumar S, Bordán Z, Cherian-Shaw M, Kovács-Kása A, MacDonald JA, Fulton DJR, Erdődi F, Verin AD. Bátori R, et al. J Cell Physiol. 2019 May;234(5):5863-5879. doi: 10.1002/jcp.26419. Epub 2018 Dec 17. J Cell Physiol. 2019. PMID: 29271489 Free PMC article. - Extracellular adenosine enhances pulmonary artery vasa vasorum endothelial cell barrier function via Gi/ELMO1/Rac1/PKA-dependent signaling mechanisms.
Verin AD, Batori R, Kovacs-Kasa A, Cherian-Shaw M, Kumar S, Czikora I, Karoor V, Strassheim D, Stenmark KR, Gerasimovskaya EV. Verin AD, et al. Am J Physiol Cell Physiol. 2020 Jul 1;319(1):C183-C193. doi: 10.1152/ajpcell.00505.2019. Epub 2020 May 20. Am J Physiol Cell Physiol. 2020. PMID: 32432925 Free PMC article. - Animal models of airway diseases.
Thompson LF, Picher M, Blackburn MR. Thompson LF, et al. Subcell Biochem. 2011;55:195-234. doi: 10.1007/978-94-007-1217-1_8. Subcell Biochem. 2011. PMID: 21560049 Free PMC article. Review. - Purinergic Regulation of Endothelial Barrier Function.
Aslam M, Gündüz D, Troidl C, Heger J, Hamm CW, Schulz R. Aslam M, et al. Int J Mol Sci. 2021 Jan 26;22(3):1207. doi: 10.3390/ijms22031207. Int J Mol Sci. 2021. PMID: 33530557 Free PMC article. Review.
References
- Adkins WK, Barnard JW, May S, Seibert AF, Haynes J, Taylor AE. Compounds that increase cAMP prevent ischemia-reperfusion pulmonary capillary injury. J Appl Physiol 72: 492–497, 1992 - PubMed
- Adkins WK, Barnard JW, Moore TM, Allison RC, Prasad VR, Taylor AE. Adenosine prevents PMA-induced lung injury via an A2 receptor mechanism. J Appl Physiol 74: 982–988, 1993 - PubMed
- Allison RC, Hernandez EM, Prasad VR, Grisham MB, Taylor AE. Protective effects of O2 radical scavengers and adenosine in PMA-induced lung injury. J Appl Physiol 64: 2175–2182, 1988 - PubMed
- Baumer Y, Drenckhahn D, Waschke J. cAMP induced Rac 1-mediated cytoskeletal reorganization in microvascular endothelium. Histochem Cell Biol 129: 765–778, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials