Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis - PubMed (original) (raw)
Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis
Marcel Mettlen et al. J Cell Biol. 2010.
Abstract
Clathrin-mediated endocytosis of surface receptors and their bound ligands (i.e., cargo) is highly regulated, including by the cargo itself. One of the possible sources of the observed heterogeneous dynamics of clathrin-coated pits (CCPs) might be the different cargo content. Consistent with this, we show that CCP size and dynamic behavior varies with low density lipoprotein receptor (LDLR) expression levels in a manner dependent on the LDLR-specific adaptors, Dab2 and ARH. In Dab2-mCherry-expressing cells, varying LDLR expression leads to a progressive increase in CCP size and to the appearance of nonterminal endocytic events. In LDLR and ARH-mCherry-expressing cells in addition to an increase in CCP size, turnover of abortive CCPs increases, and the rate of CCP maturation decreases. Altogether, our results underscore the highly dynamic and cargo-responsive nature of CCP assembly and suggest that the observed heterogeneity is, in part, related to compositional differences (e.g., cargo and adaptors) between CCPs.
Figures
Figure 1.
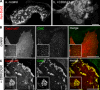
Dab2/LDLRs regulate CCP size. BSC1 cells stably expressing LCa-EGFP were transfected with Dab2-mCherry and infected with adenoviruses coding for a tet-repressible LDLR expression system. Cells were cultured in the presence of decreasing concentrations of tet, fixed/permeabilized, and observed by epifluorescence or electron microscopy. [tet]: (A–D) 1,000 ng/ml, no CD8/LDLR; (E–H): 25 ng/ml, low CD8/LDLR; arrowheads: normal-sized CCPs; arrows: enlarged CCPs (I–L): 0 ng/ml, high CD8/LDLR. Bars, 10 µm. (D, H, and L) Encircled areas indicate clathrin lattices at the ventral membrane of BSC-1 cells. Insets show normal-sized CCPs in each condition.
Figure 2.
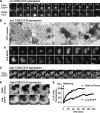
Requirements for Dab2/LDLR-induced GCCS formation. BSC1-wt cells were transfected with either myristoylated Dab2-mCherry (A) or Dab2-p67-mCherry (B and C) and were infected with adenoviruses coding for CD8/LDLR as indicated. Cells were fixed, permeabilized, immunolabeled in green for clathrin heavy chain (CHC; B and C), and observed by epifluorescence. Bars, 10 µm.
Figure 3.
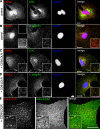
GCCSs are dynamic and, at moderate CD8/LDLR levels, generate nonterminal endocytic events. BSC1 cells were cultured as described in Fig. 1 and observed by dual-color TIRF-FM (A, Bb, C), electron microscopy (Ba), or spinning disk confocal microscopy (D). (A) Montage shows lifetime of a CCP. Arrowhead indicates nonterminal event. (B) In the presence of mild CD8/LDLR expression, CCPs tend to cluster and undergo nonterminal endocytic events. (C) GCCSs are dynamic and change morphology. (D) A small area in GCCSs has been bleached (indicated by square) and FRAP was recorded and quantified. Bars: (A) 400 nm; (Ba) 200 nm; (Bb) 600 nm; (C and D) 2 µm.
Figure 4.
ARH localization is cargo dependent. BSC1 cells stably expressing ARH-mCherry were infected with adenoviruses coding for CD8/LDLR and cultured in the presence (A and B) or absence (C and D) of tet. Cells were then fixed, permeabilized, immunolabeled as indicated, and observed by epifluorescence. Insets show enlarged areas. (E) BSC1 cells coexpressing Dab2-mCherry, ARH-EGFP, and CD8/LDLR were fixed and visualized by spinning disk confocal microscopy. Bars, 10 µm.
Figure 5.
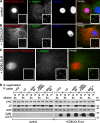
Requirements for ARH targeting to CCPs. BSC1 cells stably expressing ARH F259A-mCherry (A and B) or ARH S117Y;F165A-mCherry (C) were left untreated (A) or infected with adenoviruses coding for CD8/LDLR (B and C). Cells were then fixed, permeabilized, immunolabeled as indicated, and observed by epifluorescence. Bars, 10 µm. (D) Indicated cell lines were cultured as previously described and analyzed by Western blot after subcellular fractionation. S: cytosolic fraction (diluted 4x); P: membrane fraction. Actin served as loading control. The cell fractionation experiment has been repeated at least three times and a typical experiment is shown.
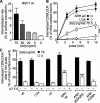
Figure 6.
ARH partially rescues saturated LDLR uptake. (A) BSC1-wt cells infected with adenovirus coding for CD8/LDLR were cultured in the presence of the indicated concentrations of tet and the rate of anti-CD8 antibody internalization relative to surface bound ligand was measured at 37°C, as described in Materials and methods. (B) Time course of single round internalization of prebound anti-CD8 antibody in BSC1 cells expressing EGFP-CLCa (○, •) or mCherry-ARH (□, ■). Shown are means (±SD) of at least three independent experiments. (C) BSC-wt cells or cells stably expressing LCa-EGFP, σ2-EGFP, ARH-wt-, ARH-F259A-, or ARH-252A255-mCherry were treated (+ µ2 k.d.) or not with µ2 siRNA to reduce AP2 levels, infected with CD8/LDLR adenovirus, and incubated with or without tet as indicated. The mean efficiency CD8/LDLR uptake as determined in at least two independent experiments is shown. Of note: to follow basal endocytic uptake of CD8, minimal exogenous expression of CD8/LDLR was necessary (i.e., overnight culture in the presence of 75 ng/ml tet). ***, confidence levels of P < 0.001 relative to wt control, 0 tet.
Figure 7.
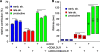
CD8/LDLRs prolong the lifetimes of productive CCPs. (A) TIR-FM time-lapse movies have been recorded and analyzed as described previously (Loerke et al., 2009). (A) Relative contributions and (B) lifetimes of CCP subpopulations labeled by LCa-EGFP are shown. Error bars represent cell-to-cell variation; the height of the lifetime bars in B denotes the range around the mean lifetime that contains 50% of the data. Brackets indicate confidence levels P < 10−8 (Kolmogorov-Smirnov test). The number of CCP trajectories (n) and cells (k) for each condition are: LCa-EGFP control (n = 100,823; k = 65); LCa-EGFP + CD8/LDLR (n = 70,955; k = 38); LCa-EGFP + ARH + CD8/LDLR (n = 23,394; k = 17).
Figure 8.
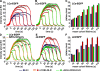
CD8/LDLR and adaptors affect CCP size. TIR-FM time-lapse movies have been recorded under various conditions, following the indicated fluorescently tagged protein. CCP cohorts of given lifetimes have been binned and their intensity profiles (A, B, D, and E) determined as a measure for CCP growth. The number of CCP trajectories for each condition, which ranges from 626 to 20,000, is shown in
Table S1
. The maximum plateau level of the LCa-EGFP (C) or σ2-EGFP (F) is plotted against the mean cohort lifetime for the indicated cells.
Similar articles
- The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH.
Maurer ME, Cooper JA. Maurer ME, et al. J Cell Sci. 2006 Oct 15;119(Pt 20):4235-46. doi: 10.1242/jcs.03217. Epub 2006 Sep 19. J Cell Sci. 2006. PMID: 16984970 - A single common portal for clathrin-mediated endocytosis of distinct cargo governed by cargo-selective adaptors.
Keyel PA, Mishra SK, Roth R, Heuser JE, Watkins SC, Traub LM. Keyel PA, et al. Mol Biol Cell. 2006 Oct;17(10):4300-17. doi: 10.1091/mbc.e06-05-0421. Epub 2006 Jul 26. Mol Biol Cell. 2006. PMID: 16870701 Free PMC article. - Cargo regulates clathrin-coated pit dynamics.
Puthenveedu MA, von Zastrow M. Puthenveedu MA, et al. Cell. 2006 Oct 6;127(1):113-24. doi: 10.1016/j.cell.2006.08.035. Cell. 2006. PMID: 17018281 - Understanding living clathrin-coated pits.
Rappoport JZ, Simon SM, Benmerah A. Rappoport JZ, et al. Traffic. 2004 May;5(5):327-37. doi: 10.1111/j.1398-9219.2004.00187.x. Traffic. 2004. PMID: 15086782 Review. - Cargo recognition during clathrin-mediated endocytosis: a team effort.
Sorkin A. Sorkin A. Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
Cited by
- Expanding proteostasis by membrane trafficking networks.
Hutt DM, Balch WE. Hutt DM, et al. Cold Spring Harb Perspect Biol. 2013 Jul 1;5(7):a013383. doi: 10.1101/cshperspect.a013383. Cold Spring Harb Perspect Biol. 2013. PMID: 23426524 Free PMC article. Review. - Plasma membrane domains enriched in cortical endoplasmic reticulum function as membrane protein trafficking hubs.
Fox PD, Haberkorn CJ, Weigel AV, Higgins JL, Akin EJ, Kennedy MJ, Krapf D, Tamkun MM. Fox PD, et al. Mol Biol Cell. 2013 Sep;24(17):2703-13. doi: 10.1091/mbc.E12-12-0895. Epub 2013 Jul 17. Mol Biol Cell. 2013. PMID: 23864710 Free PMC article. - Analysis of yeast endocytic site formation and maturation through a regulatory transition point.
Carroll SY, Stimpson HE, Weinberg J, Toret CP, Sun Y, Drubin DG. Carroll SY, et al. Mol Biol Cell. 2012 Feb;23(4):657-68. doi: 10.1091/mbc.E11-02-0108. Epub 2011 Dec 21. Mol Biol Cell. 2012. PMID: 22190733 Free PMC article. - Mechanistic divergences of endocytic clathrin-coated vesicle formation in mammals, yeasts and plants.
Johnson A. Johnson A. J Cell Sci. 2024 Aug 15;137(16):jcs261847. doi: 10.1242/jcs.261847. Epub 2024 Aug 20. J Cell Sci. 2024. PMID: 39161994 Free PMC article. Review. - ADAP1 promotes latent HIV-1 reactivation by selectively tuning KRAS-ERK-AP-1 T cell signaling-transcriptional axis.
Ramirez NP, Lee J, Zheng Y, Li L, Dennis B, Chen D, Challa A, Planelles V, Westover KD, Alto NM, D'Orso I. Ramirez NP, et al. Nat Commun. 2022 Mar 1;13(1):1109. doi: 10.1038/s41467-022-28772-0. Nat Commun. 2022. PMID: 35232997 Free PMC article.
References
- Arca M., Zuliani G., Wilund K., Campagna F., Fellin R., Bertolini S., Calandra S., Ricci G., Glorioso N., Maioli M., et al. 2002. Autosomal recessive hypercholesterolaemia in Sardinia, Italy, and mutations in ARH: a clinical and molecular genetic analysis. Lancet. 359:841–847 10.1016/S0140-6736(02)07955-2 - DOI - PubMed
- Edeling M.A., Mishra S.K., Keyel P.A., Steinhauser A.L., Collins B.M., Roth R., Heuser J.E., Owen D.J., Traub L.M. 2006. Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev. Cell. 10:329–342 10.1016/j.devcel.2006.01.016 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 MH061345/MH/NIMH NIH HHS/United States
- MH61345/MH/NIMH NIH HHS/United States
- R01 MH061345/MH/NIMH NIH HHS/United States
- GM73165/GM/NIGMS NIH HHS/United States
- R01 GM073165/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases