Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study - PubMed (original) (raw)
Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study
Wayne A Ray et al. Ann Intern Med. 2010.
Abstract
Background: Proton-pump inhibitors (PPIs) and clopidogrel are frequently coprescribed, although the benefits and harms of their concurrent use are unclear.
Objective: To examine the association between concurrent use of PPIs and clopidogrel and the risks for hospitalizations for gastroduodenal bleeding and serious cardiovascular disease.
Design: Retrospective cohort study using automated data to identify patients who received clopidogrel between 1999 through 2005 after hospitalization for coronary heart disease.
Setting: Tennessee Medicaid program.
Patients: 20,596 patients (including 7593 concurrent users of clopidogrel and PPIs) hospitalized for myocardial infarction, coronary artery revascularization, or unstable angina pectoris.
Measurements: Baseline and follow-up drug use was assessed from automated records of dispensed prescriptions. Primary outcomes were hospitalizations for gastroduodenal bleeding and serious cardiovascular disease (fatal or nonfatal myocardial infarction or sudden cardiac death, stroke, or other cardiovascular death).
Results: Pantoprazole and omeprazole accounted for 62% and 9% of concurrent PPI use, respectively. Adjusted incidence of hospitalization for gastroduodenal bleeding in concurrent PPI users was 50% lower than that in nonusers (hazard ratio, 0.50 [95% CI, 0.39 to 0.65]). For patients at highest risk for bleeding, PPI use was associated with an absolute reduction of 28.5 (CI, 11.7 to 36.9) hospitalizations for gastroduodenal bleeding per 1000 person-years. The hazard ratio associated with concurrent PPI use for risk for serious cardiovascular disease was 0.99 (CI, 0.82 to 1.19) for the entire cohort and 1.01 (CI, 0.76 to 1.34) for the subgroup of patients who had percutaneous coronary interventions with stenting during the qualifying hospitalization.
Limitations: Unmeasured confounding and misclassification of exposure (no information on adherence or over-the-counter use of drugs) and end points (not confirmed by medical record review) were possible. Because many patients entered the cohort from hospitals with relatively few cohort members, the analysis relied on the assumption that after adjustment for observed covariates, PPI users from one such hospital could be compared with nonusers from a different hospital.
Conclusion: In patients with serious coronary heart disease treated with clopidogrel, concurrent PPI use was associated with reduced incidence of hospitalizations for gastroduodenal bleeding. The corresponding point estimate for serious cardiovascular disease was not increased; however, the 95% CI included a clinically important increased risk.
Primary funding source: Agency for Healthcare Research and Quality and National Heart, Lung, and Blood Institute.
Figures
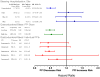
Figure 1
Relative risk of bleeding endpoints for current clopidogrel users according to use of individual PPIs and PPI dose. Reference category is nonusers of any PPI. PY is person-years are person years of PPI use, events is number of cases of serious cardiovascular disease. The sum for individual drugs and doses does not equal total use because the former exclude persons with use of more than one PPI. HR is the adjusted hazard ratio, CI, confidence interval. The high dose cutpoints were: esomeprazole >40mg, omeprazole >20mg, pantoprazole >40mg, rabeprazole >20mg, and lanzoprazole >30mg.
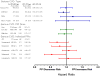
Figure 2
Relative risk of serious cardiovascular disease endpoint (nonfatal or fatal myocardial infarction, stroke, or other cardiovascular death) for current clopidogrel users according to use of individual PPIs and PPI dose. Reference category is nonusers of any PPI. PY is person-years are person years of PPI use, events is number of cases of serious cardiovascular disease. The sum for individual drugs and doses does not equal total use because the former exclude persons with use of more than one PPI. HR is the adjusted hazard ratio, CI, confidence interval. The high dose cutpoints were: esomeprazole >40mg, omeprazole >20mg, pantoprazole >40mg, rabeprazole >20mg, and lanzoprazole >30mg.
Comment in
- Propensity score adjustment with multilevel data: setting your sites on decreasing selection bias.
Griswold ME, Localio AR, Mulrow C. Griswold ME, et al. Ann Intern Med. 2010 Mar 16;152(6):393-5. doi: 10.7326/0003-4819-152-6-201003160-00010. Ann Intern Med. 2010. PMID: 20231571 No abstract available.
Similar articles
- Effect of individual proton pump inhibitors on cardiovascular events in patients treated with clopidogrel following coronary stenting: results from the Ibaraki Cardiac Assessment Study Registry.
Aihara H, Sato A, Takeyasu N, Nishina H, Hoshi T, Akiyama D, Kakefuda Y, Watabe H, Aonuma K; ICAS Registry Investigators. Aihara H, et al. Catheter Cardiovasc Interv. 2012 Oct 1;80(4):556-63. doi: 10.1002/ccd.23327. Epub 2012 Jan 10. Catheter Cardiovasc Interv. 2012. PMID: 22234956 - One-year clinical outcome in patients with acute coronary syndrome treated with concomitant use of clopidogrel and proton pump inhibitors: results from a regional cohort study.
Ortolani P, Marino M, Marzocchi A, De Palma R, Branzi A. Ortolani P, et al. J Cardiovasc Med (Hagerstown). 2012 Dec;13(12):783-9. doi: 10.2459/JCM.0b013e3283416b6b. J Cardiovasc Med (Hagerstown). 2012. PMID: 21252697 - Clopidogrel and proton pump inhibitors--where do we stand in 2012?
Drepper MD, Spahr L, Frossard JL. Drepper MD, et al. World J Gastroenterol. 2012 May 14;18(18):2161-71. doi: 10.3748/wjg.v18.i18.2161. World J Gastroenterol. 2012. PMID: 22611308 Free PMC article. Review. - Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials.
O'Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, Michelson AD, Hautvast RW, Ver Lee PN, Close SL, Shen L, Mega JL, Sabatine MS, Wiviott SD. O'Donoghue ML, et al. Lancet. 2009 Sep 19;374(9694):989-997. doi: 10.1016/S0140-6736(09)61525-7. Epub 2009 Aug 31. Lancet. 2009. PMID: 19726078 Review.
Cited by
- PPIs Are Not Responsible for Elevating Cardiovascular Risk in Patients on Clopidogrel-A Systematic Review and Meta-Analysis.
Demcsák A, Lantos T, Bálint ER, Hartmann P, Vincze Á, Bajor J, Czopf L, Alizadeh H, Gyöngyi Z, Márta K, Mikó A, Szakács Z, Pécsi D, Hegyi P, Szabó IL. Demcsák A, et al. Front Physiol. 2018 Nov 19;9:1550. doi: 10.3389/fphys.2018.01550. eCollection 2018. Front Physiol. 2018. PMID: 30510515 Free PMC article. - Proton pump inhibitors and clopidogrel: an association to avoid?
D'Ugo E, Rossi S, De Caterina R. D'Ugo E, et al. Intern Emerg Med. 2014 Feb;9(1):11-22. doi: 10.1007/s11739-013-1000-4. Epub 2013 Sep 13. Intern Emerg Med. 2014. PMID: 24030523 Review. - Role of antiplatelet therapy in secondary prevention of acute coronary syndrome.
Pankert M, Quilici J, Cuisset T. Pankert M, et al. J Cardiovasc Transl Res. 2012 Feb;5(1):41-51. doi: 10.1007/s12265-011-9329-4. Epub 2011 Nov 6. J Cardiovasc Transl Res. 2012. PMID: 22057689 Review. - Effects of omeprazole or pantoprazole on platelet function in non-ST-segment elevation acute coronary syndrome patients receiving clopidogrel.
Gu RX, Wang XZ, Li J, Deng J, Li XX, Wang J. Gu RX, et al. Mil Med Res. 2016 Dec 15;3:38. doi: 10.1186/s40779-016-0107-0. eCollection 2016. Mil Med Res. 2016. PMID: 28018669 Free PMC article. - Individualised PPI prescription in patients on combination antiplatelet therapy and upper gastrointestinal events after percutaneous coronary intervention: a cohort study.
Häuptle R, Weilenmann D, Schneider T, Haile SR, Ammann P, Knellwolf C, Borovicka J. Häuptle R, et al. Wien Med Wochenschr. 2012 Feb;162(3-4):67-73. doi: 10.1007/s10354-012-0056-5. Wien Med Wochenschr. 2012. PMID: 22476595
References
- Gurbel PA, Lau WC, Tantry US. Omeprazole. A possible new candidate influencing the antiplatelet effect of clopidogrel. J Am Coll Surg. 2008;51:261–63. - PubMed
- Bhatt DL, Fox KAA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Eng J Med. 2006;354:1706–17. - PubMed
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. - PubMed
- Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FKL, Furberg CD, et al. ACCF/ACG/AHA 2008 Expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2008 Oct;15:1–16. - PubMed
- Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL081707/HL/NHLBI NIH HHS/United States
- R56 HL081707/HL/NHLBI NIH HHS/United States
- HS1-16974/HS/AHRQ HHS/United States
- R01 HL081707/HL/NHLBI NIH HHS/United States
- R01 HL096844/HL/NHLBI NIH HHS/United States
- R01 HL081707-07/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical