V3D enables real-time 3D visualization and quantitative analysis of large-scale biological image data sets - PubMed (original) (raw)
V3D enables real-time 3D visualization and quantitative analysis of large-scale biological image data sets
Hanchuan Peng et al. Nat Biotechnol. 2010 Apr.
Abstract
The V3D system provides three-dimensional (3D) visualization of gigabyte-sized microscopy image stacks in real time on current laptops and desktops. V3D streamlines the online analysis, measurement and proofreading of complicated image patterns by combining ergonomic functions for selecting a location in an image directly in 3D space and for displaying biological measurements, such as from fluorescent probes, using the overlaid surface objects. V3D runs on all major computer platforms and can be enhanced by software plug-ins to address specific biological problems. To demonstrate this extensibility, we built a V3D-based application, V3D-Neuron, to reconstruct complex 3D neuronal structures from high-resolution brain images. V3D-Neuron can precisely digitize the morphology of a single neuron in a fruitfly brain in minutes, with about a 17-fold improvement in reliability and tenfold savings in time compared with other neuron reconstruction tools. Using V3D-Neuron, we demonstrate the feasibility of building a 3D digital atlas of neurite tracts in the fruitfly brain.
Figures
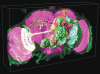
Figure 1
V3D visualization. (a) The use of V3D in visualizing a 3D digital model of a fruit fly brain. Magenta voxels: the 3D volumetric image of a fruit fly brain; green voxels: a 3D GAL4 neurite pattern; colored surface objects of irregular shapes: digital models of various brain compartments; colored tree-like surface objects: 3D reconstructed neurons. (b) The volumetric image rendering speed of V3D visualization engine under synchronous and asynchronous modes. For each image size, both the peak speed (green and yellow bars) and the respective standard deviation (black line-ranges) of at least 10 speed-test trials are shown. The tests were done on a 64bit Redhat Linux machine with a GTX280 graphics card. (c) V3D hierarchical visualization and analysis. Local 3D viewers of different brain regions can be conveniently initialized from the global viewer. Local viewers can have their own color maps and surface objects independent of the global viewer. They can also be used to analyze sub-volumes of an image separately.
Figure 1
V3D visualization. (a) The use of V3D in visualizing a 3D digital model of a fruit fly brain. Magenta voxels: the 3D volumetric image of a fruit fly brain; green voxels: a 3D GAL4 neurite pattern; colored surface objects of irregular shapes: digital models of various brain compartments; colored tree-like surface objects: 3D reconstructed neurons. (b) The volumetric image rendering speed of V3D visualization engine under synchronous and asynchronous modes. For each image size, both the peak speed (green and yellow bars) and the respective standard deviation (black line-ranges) of at least 10 speed-test trials are shown. The tests were done on a 64bit Redhat Linux machine with a GTX280 graphics card. (c) V3D hierarchical visualization and analysis. Local 3D viewers of different brain regions can be conveniently initialized from the global viewer. Local viewers can have their own color maps and surface objects independent of the global viewer. They can also be used to analyze sub-volumes of an image separately.
Figure 1
V3D visualization. (a) The use of V3D in visualizing a 3D digital model of a fruit fly brain. Magenta voxels: the 3D volumetric image of a fruit fly brain; green voxels: a 3D GAL4 neurite pattern; colored surface objects of irregular shapes: digital models of various brain compartments; colored tree-like surface objects: 3D reconstructed neurons. (b) The volumetric image rendering speed of V3D visualization engine under synchronous and asynchronous modes. For each image size, both the peak speed (green and yellow bars) and the respective standard deviation (black line-ranges) of at least 10 speed-test trials are shown. The tests were done on a 64bit Redhat Linux machine with a GTX280 graphics card. (c) V3D hierarchical visualization and analysis. Local 3D viewers of different brain regions can be conveniently initialized from the global viewer. Local viewers can have their own color maps and surface objects independent of the global viewer. They can also be used to analyze sub-volumes of an image separately.
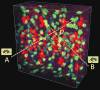
Figure 2
3D pinpointing methods of V3D. (a) 3D pinpointing using 2 mouse-clicks. The color image is a 3D confocal image of neurons, fluorescently tagged for three different transcriptional factors (repo, eve, and _hb_9) at the same time, in a fruit fly embryo. A and B: non-parallel rays generated at two viewing angles, corresponding to two mouse-clicks; p: the estimated 3D location that is closest to both A and B. (b) 3D pinpointing using 1 mouse-click. _p_1 to pN: the progressively estimated centers of mass; _R_1 to RN: the progressively smaller intervals to estimate _p_1 to pN.
Figure 2
3D pinpointing methods of V3D. (a) 3D pinpointing using 2 mouse-clicks. The color image is a 3D confocal image of neurons, fluorescently tagged for three different transcriptional factors (repo, eve, and _hb_9) at the same time, in a fruit fly embryo. A and B: non-parallel rays generated at two viewing angles, corresponding to two mouse-clicks; p: the estimated 3D location that is closest to both A and B. (b) 3D pinpointing using 1 mouse-click. _p_1 to pN: the progressively estimated centers of mass; _R_1 to RN: the progressively smaller intervals to estimate _p_1 to pN.
Figure 3
Quantitative measuring of 3D gene expression gradient in a C. elegans confocal image. Green voxels: myo3:GFP-tagged body wall muscle cells; blue voxels: DAPI (4,6-diamidino-2-phenylindole)-tagged nuclei for the entire animal; colored spheres: pinpointed markers; colored line-segments: the line-indicator for measuring along different directions and with different starting and ending locations; line profile graph: the channel-by-channel display of the voxel intensity along a line segment.
Figure 4
V3D-Neuron tracing. (a) Pinpointing terminals of a fruit fly neuron. 3D image: a GFP-tagged neuron produced via twin-spot MARCM; colored spheres: markers defined for the tips of this neuron. (b) 3D reconstructed neuron produced by V3D-Neuron. Colored segments: the automatically reconstructed neurite structures. (c) The skeleton view of the 3D reconstructed neuron.
Figure 4
V3D-Neuron tracing. (a) Pinpointing terminals of a fruit fly neuron. 3D image: a GFP-tagged neuron produced via twin-spot MARCM; colored spheres: markers defined for the tips of this neuron. (b) 3D reconstructed neuron produced by V3D-Neuron. Colored segments: the automatically reconstructed neurite structures. (c) The skeleton view of the 3D reconstructed neuron.
Figure 4
V3D-Neuron tracing. (a) Pinpointing terminals of a fruit fly neuron. 3D image: a GFP-tagged neuron produced via twin-spot MARCM; colored spheres: markers defined for the tips of this neuron. (b) 3D reconstructed neuron produced by V3D-Neuron. Colored segments: the automatically reconstructed neurite structures. (c) The skeleton view of the 3D reconstructed neuron.
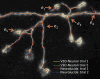
Figure 5
The accuracy of V3D-Neuron reconstructions compared with manual reconstructions. (a) Inconsistency of independent trials of reconstructions. _e_1, _e_2, _e_3, _e_4: examples of the obvious inconsistent parts in manual reconstructions; _e_5: an example of the inconsistent region in V3D-Neuron reconstructions. (b) The spatial divergence of reconstructed neurons using different methods, each with two independent runs. Also shown in the legend are the average and the standard deviation of the spatial divergence (see Methods) of all neurons. (c) The percent of the neuron structure that noticeably varies in independent reconstructions. Also shown in the legend are the average and the standard deviation of this score over all neurons. SSD: substantial spatial distance.
Figure 5
The accuracy of V3D-Neuron reconstructions compared with manual reconstructions. (a) Inconsistency of independent trials of reconstructions. _e_1, _e_2, _e_3, _e_4: examples of the obvious inconsistent parts in manual reconstructions; _e_5: an example of the inconsistent region in V3D-Neuron reconstructions. (b) The spatial divergence of reconstructed neurons using different methods, each with two independent runs. Also shown in the legend are the average and the standard deviation of the spatial divergence (see Methods) of all neurons. (c) The percent of the neuron structure that noticeably varies in independent reconstructions. Also shown in the legend are the average and the standard deviation of this score over all neurons. SSD: substantial spatial distance.
Figure 5
The accuracy of V3D-Neuron reconstructions compared with manual reconstructions. (a) Inconsistency of independent trials of reconstructions. _e_1, _e_2, _e_3, _e_4: examples of the obvious inconsistent parts in manual reconstructions; _e_5: an example of the inconsistent region in V3D-Neuron reconstructions. (b) The spatial divergence of reconstructed neurons using different methods, each with two independent runs. Also shown in the legend are the average and the standard deviation of the spatial divergence (see Methods) of all neurons. (c) The percent of the neuron structure that noticeably varies in independent reconstructions. Also shown in the legend are the average and the standard deviation of this score over all neurons. SSD: substantial spatial distance.
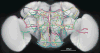
Figure 6
An atlas of stereotyped neurite tracts in a fruit fly brain. (a) Statistical models of the 3D reconstructed neurite tracts. Grayscale image: a fruit fly brain; each colored tubular structure: the average of multiple neurite tracts reconstructed from images of the same GAL4 line. The width of the each tract equals twice of the spatial variation of the respective group of reconstructions. (b) Distribution of the spatial variation of all neurite tracts.
Figure 6
An atlas of stereotyped neurite tracts in a fruit fly brain. (a) Statistical models of the 3D reconstructed neurite tracts. Grayscale image: a fruit fly brain; each colored tubular structure: the average of multiple neurite tracts reconstructed from images of the same GAL4 line. The width of the each tract equals twice of the spatial variation of the respective group of reconstructions. (b) Distribution of the spatial variation of all neurite tracts.
Comment in
- Connecting the dots in 3D.
Evanko D. Evanko D. Nat Methods. 2010 May;7(5):344-5. doi: 10.1038/nmeth0510-344a. Nat Methods. 2010. PMID: 20440880 No abstract available.
Similar articles
- Interviews3D: a platform for interactive handling of massive data sets.
Brüderlin B, Heyer M, Pfützner S. Brüderlin B, et al. IEEE Comput Graph Appl. 2007 Nov-Dec;27(6):48-59. doi: 10.1109/mcg.2007.153. IEEE Comput Graph Appl. 2007. PMID: 18027797 Review. - Interactive visualization of multiresolution image stacks in 3D.
Trotts I, Mikula S, Jones EG. Trotts I, et al. Neuroimage. 2007 Apr 15;35(3):1038-43. doi: 10.1016/j.neuroimage.2007.01.013. Epub 2007 Jan 27. Neuroimage. 2007. PMID: 17336095 Free PMC article. - Structural shape prototypes for the automatic classification of 3D objects.
Marini S, Spagnuolo M, Falcidieno B. Marini S, et al. IEEE Comput Graph Appl. 2007 Jul-Aug;27(4):28-37. doi: 10.1109/mcg.2007.89. IEEE Comput Graph Appl. 2007. PMID: 17713232 Review. No abstract available. - 3D documents.
Fellner DW, Saupe D, Krottmaier H. Fellner DW, et al. IEEE Comput Graph Appl. 2007 Jul-Aug;27(4):20-1. doi: 10.1109/mcg.2007.83. IEEE Comput Graph Appl. 2007. PMID: 17713230 No abstract available. - Content-based 3D object retrieval.
Bustos B, Keim D, Saupe D, Schreck T. Bustos B, et al. IEEE Comput Graph Appl. 2007 Jul-Aug;27(4):22-7. doi: 10.1109/mcg.2007.80. IEEE Comput Graph Appl. 2007. PMID: 17713231 Review. No abstract available.
Cited by
- Software tools for 3D nuclei segmentation and quantitative analysis in multicellular aggregates.
Piccinini F, Balassa T, Carbonaro A, Diosdi A, Toth T, Moshkov N, Tasnadi EA, Horvath P. Piccinini F, et al. Comput Struct Biotechnol J. 2020 Jun 3;18:1287-1300. doi: 10.1016/j.csbj.2020.05.022. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32612752 Free PMC article. Review. - Structure tensor analysis of serial optical coherence scanner images for mapping fiber orientations and tractography in the brain.
Wang H, Lenglet C, Akkin T. Wang H, et al. J Biomed Opt. 2015 Mar;20(3):036003. doi: 10.1117/1.JBO.20.3.036003. J Biomed Opt. 2015. PMID: 25741662 Free PMC article. - BioImageXD: an open, general-purpose and high-throughput image-processing platform.
Kankaanpää P, Paavolainen L, Tiitta S, Karjalainen M, Päivärinne J, Nieminen J, Marjomäki V, Heino J, White DJ. Kankaanpää P, et al. Nat Methods. 2012 Jun 28;9(7):683-9. doi: 10.1038/nmeth.2047. Nat Methods. 2012. PMID: 22743773 - SOAX: a software for quantification of 3D biopolymer networks.
Xu T, Vavylonis D, Tsai FC, Koenderink GH, Nie W, Yusuf E, I-Ju Lee, Wu JQ, Huang X. Xu T, et al. Sci Rep. 2015 Mar 13;5:9081. doi: 10.1038/srep09081. Sci Rep. 2015. PMID: 25765313 Free PMC article. - Density Visualization Pipeline: A Tool for Cellular and Network Density Visualization and Analysis.
Grein S, Qi G, Queisser G. Grein S, et al. Front Comput Neurosci. 2020 Jun 26;14:42. doi: 10.3389/fncom.2020.00042. eCollection 2020. Front Comput Neurosci. 2020. PMID: 32676020 Free PMC article.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11(7):36–42.
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera-a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. - PubMed
- Schroeder W, Martin K, Lorensen B. Visualization Toolkit: An Object-Oriented Approach to 3D Graphics. 4. (Hardcover), Kitware, Inc; Clifton Park, NY: 2006.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources