Involvement of oxidative stress in the relapse of acute myeloid leukemia - PubMed (original) (raw)
Involvement of oxidative stress in the relapse of acute myeloid leukemia
Fu-Ling Zhou et al. J Biol Chem. 2010.
Abstract
The aims of the present study were to determine the level of oxidative stress and the salient factors leading to the relapse of acute myeloid leukemia (AML). Oxidative stress-related parameters and the expressions of specific genes were monitored in 102 cases of AML during a pretreatment period from a primary status to a relapse status. In addition, age-matched healthy subjects were classified as controls. The activities of adenosine deaminase and xanthine oxidase were higher in the relapse condition, whereas those of glutathione peroxidase, monoamine oxidase, and superoxide dismutase, and the total antioxidant capacity (T-AOC) were lower in the primary condition and in controls. Of particular note, levels of advanced oxidation protein products, malondialdehyde, and 8-hydroxydeoxyguanosine were also significantly higher in relapse patients. Furthermore, real-time PCR with SYBR Green revealed that the expression levels of human thioredoxin (TRX) and indoleamine 2,3-dioxygenase were increased in relapse patients. Pearson correlation analysis revealed that the T-AOC was positively correlated with GSH but negatively correlated with 8-OHdG, TRX, and indoleamine 2,3-dioxygenase. Linear regression showed that a low T-AOC and up-regulated TRX expression were the independent factors correlated with relapse. A strong association between oxidative stress and the incidence of disease relapse was observed, which has potential prognosis implications. These results indicate that oxidative stress is a crucial feature of AML and probably affects the development and relapse of AML.
Figures
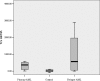
FIGURE 1.
TRX expression in the different groups.
Similar articles
- Jab1/Csn5-Thioredoxin Signaling in Relapsed Acute Monocytic Leukemia under Oxidative Stress.
Zhou F, Pan Y, Wei Y, Zhang R, Bai G, Shen Q, Meng S, Le XF, Andreeff M, Claret FX. Zhou F, et al. Clin Cancer Res. 2017 Aug 1;23(15):4450-4461. doi: 10.1158/1078-0432.CCR-16-2426. Epub 2017 Mar 7. Clin Cancer Res. 2017. PMID: 28270496 Free PMC article. - Correlation of Serum Oxidative Stress with the Effect of Initial Induction Chemotherapy in Acute Myeloid Leukemia.
Xie S, Xiao T, Wang W, Guo X. Xie S, et al. Clin Lab. 2024 Nov 1;70(11). doi: 10.7754/Clin.Lab.2024.240410. Clin Lab. 2024. PMID: 39506599 - Oxidative Stress in Tunisian Patients With Acute Lymphoblastic Leukemia and Its Involvement in Leukemic Relapse.
Ben Mahmoud L, Mdhaffar M, Ghozzi H, Ammar M, Hakim A, Atheymen R, Sahnoun Z, Elloumi M, Zeghal K. Ben Mahmoud L, et al. J Pediatr Hematol Oncol. 2017 Apr;39(3):e124-e130. doi: 10.1097/MPH.0000000000000793. J Pediatr Hematol Oncol. 2017. PMID: 28306688 Free PMC article. - Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, Cu-Zn superoxide dismutase activities, total glutathione, nitric oxide, and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer.
Kaynar H, Meral M, Turhan H, Keles M, Celik G, Akcay F. Kaynar H, et al. Cancer Lett. 2005 Sep 28;227(2):133-9. doi: 10.1016/j.canlet.2004.12.005. Epub 2005 Jan 8. Cancer Lett. 2005. PMID: 16112416 - Serum glutathione peroxidase, xanthine oxidase, and superoxide dismutase activities and malondialdehyde levels in patients with Parkinson's disease.
Gökçe Çokal B, Yurtdaş M, Keskin Güler S, Güneş HN, Ataç Uçar C, Aytaç B, Durak ZE, Yoldaş TK, Durak İ, Çubukçu HC. Gökçe Çokal B, et al. Neurol Sci. 2017 Mar;38(3):425-431. doi: 10.1007/s10072-016-2782-8. Epub 2016 Nov 30. Neurol Sci. 2017. PMID: 27900485
Cited by
- Autoantibodies Against Carbonic Anhydrase I and II in Patients with Acute Myeloid Leukemia.
Menteşe A, Erkut N, Demir S, Özer Yaman S, Sümer A, Doğramacı Ş, Alver A, Sönmez M. Menteşe A, et al. Turk J Haematol. 2017 Dec 1;34(4):307-313. doi: 10.4274/tjh.2016.0341. Epub 2017 Mar 8. Turk J Haematol. 2017. PMID: 28270370 Free PMC article. - Eltrombopag modulates reactive oxygen species and decreases acute myeloid leukemia cell survival.
Kalota A, Selak MA, Garcia-Cid LA, Carroll M. Kalota A, et al. PLoS One. 2015 Apr 27;10(4):e0126691. doi: 10.1371/journal.pone.0126691. eCollection 2015. PLoS One. 2015. PMID: 25915523 Free PMC article. - Glyphosate exposure and urinary oxidative stress biomarkers in the Agricultural Health Study.
Chang VC, Andreotti G, Ospina M, Parks CG, Liu D, Shearer JJ, Rothman N, Silverman DT, Sandler DP, Calafat AM, Beane Freeman LE, Hofmann JN. Chang VC, et al. J Natl Cancer Inst. 2023 Apr 11;115(4):394-404. doi: 10.1093/jnci/djac242. J Natl Cancer Inst. 2023. PMID: 36629488 Free PMC article. - Potential of the dietary antioxidants resveratrol and curcumin in prevention and treatment of hematologic malignancies.
Kelkel M, Jacob C, Dicato M, Diederich M. Kelkel M, et al. Molecules. 2010 Oct 12;15(10):7035-74. doi: 10.3390/molecules15107035. Molecules. 2010. PMID: 20944521 Free PMC article. Review. - Jab1/Csn5-Thioredoxin Signaling in Relapsed Acute Monocytic Leukemia under Oxidative Stress.
Zhou F, Pan Y, Wei Y, Zhang R, Bai G, Shen Q, Meng S, Le XF, Andreeff M, Claret FX. Zhou F, et al. Clin Cancer Res. 2017 Aug 1;23(15):4450-4461. doi: 10.1158/1078-0432.CCR-16-2426. Epub 2017 Mar 7. Clin Cancer Res. 2017. PMID: 28270496 Free PMC article.
References
- Valko M., Rhodes C. J., Moncol J., Izakovic M., Mazur M. (2006) Chem. Biol. Interact. 160, 1–40 - PubMed
- Selmeci L., Seres L., Soós P., Székely M., Acsády G. (2008) Acta Physiol. Hung. 95, 209–218 - PubMed
- Selmeci L., Seres L., Antal M., Lukács J., Regöly-Mérei A., Acsády G. (2005) Clin. Chem. Lab. Med. 43, 294–297 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials