Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation - PubMed (original) (raw)
Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation
Jeffrey J Lochhead et al. J Cereb Blood Flow Metab. 2010 Sep.
Erratum in
- J Cereb Blood Flow Metab. 2011 Feb;31(2):790-1
Abstract
The blood-brain barrier (BBB) has a critical role in central nervous system homeostasis. Intercellular tight junction (TJ) protein complexes of the brain microvasculature limit paracellular diffusion of substances from the blood into the brain. Hypoxia and reoxygenation (HR) is a central component to numerous disease states and pathologic conditions. We have previously shown that HR can influence the permeability of the BBB as well as the critical TJ protein occludin. During HR, free radicals are produced, which may lead to oxidative stress. Using the free radical scavenger tempol (200 mg/kg, intraperitoneal), we show that oxidative stress produced during HR (6% O(2) for 1 h, followed by room air for 20 min) mediates an increase in BBB permeability in vivo using in situ brain perfusion. We also show that these changes are associated with alterations in the structure and localization of occludin. Our data indicate that oxidative stress is associated with movement of occludin away from the TJ. Furthermore, subcellular fractionation of cerebral microvessels reveals alterations in occludin oligomeric assemblies in TJ associated with plasma membrane lipid rafts. Our data suggest that pharmacological inhibition of disease states with an HR component may help preserve BBB functional integrity.
Figures
Figure 1
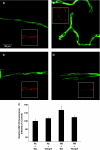
Hypoxia and reoxygenation (HR) induces hypoxia-inducible factor 1_α_ (Hif-1_α_) translocation to the nucleus. Immunofluorescence of Hif-1_α_ (green) and the nuclear dye TO-PRO-3 (blue) in brain microvessels of normoxic (Nx) plus saline (A), Nx plus tempol (B), HR plus saline (C), and HR plus tempol (D)-treated rats. Rats exposed to HR show an increase in translocation of Hif-1_α_ to the nucleus (arrow). Immunofluorescence of the endothelial-specific marker von Willebrand factor (red) is shown in the inset.
Figure 2
Oxidative stress induces expression of heat shock protein (HSP-70). Immunofluorescence of HSP-70 (green) in brain microvessels of normoxic (Nx) plus saline (Nx+S) (A), Nx plus tempol (Nx+T) (B), hypoxia–reoxygenation (HR) plus S (HR+S) (C), and HR + T (D)-treated rats. Rats exposed to HR show an increase in HSP-70 immunofluorescence. Tempol attenuated this increase. Immunofluorescence of the endothelial-specific marker von Willebrand factor (red) is shown in the inset. (E) Mean fluorescence intensity of HSP-70 in all treatment groups expressed as a percent control. *P<0.05 versus HR+T and Nx+T. P<0.01 versus Nx+S (_n_=28 to 33 microvesels per treatment group).
Figure 3
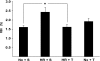
Effects of hypoxia and reoxygenation (HR) and tempol treatment on BBB permeability to 14C-sucrose. After treatment, normoxia (Nx) plus saline (S) (Nx+S), Nx plus tempol (T) (Nx+T), HR+S, and HR+T rats were anesthetized and subjected to in situ brain perfusion for 10 mins with 14C-sucrose. The amount of radioactivity in the brain versus the perfusate was expressed as Rbr%. Results are expressed as mean±s.e. *P<0.05.
Figure 4
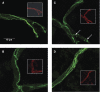
Oxidative stress induces changes in occludin localization. Immunofluorescence of occludin (green) and the endothelial-specific marker platelet-endothelial cell adhesion molecule 1 (PECAM-1) (red inset) in brain microvessels of normoxic (Nx) plus saline (A), Nx plus tempol (B), hypoxia–reoxygenation (HR) plus saline (C), and HR plus tempol (D). HR induces an increase in punctate staining of occludin (arrows), suggesting the movement of occludin away from the tight junction. Tempol prevents this increase, indicating that oxidative stress is involved in altering the cellular localization of occludin.
Figure 5
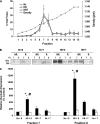
Density gradient fractionation of cerebral microvessels. (A) Profiles of protein concentrations and refractive indices (density) of cerebral microvessels after fractionation in normoxia (Nx) plus saline (S) (Nx+S), Nx plus tempol (T) (Nx+T), hypoxia–reoxygenation (HR) + S (HR+S), and HR+T. (B) Representative sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot of occludin oligomers in fractions 7 and 8 under nonreducing (NR) and reducing (R) conditions. Fractions 7 and 8 have previously been shown to be associated with the tight junction in lipid rafts at the plasma membrane. (C) Western blot analysis of occludin oligomers in fractions 7 and 8 under reducing and nonreducing conditions. Data are presented as mean±s.e. and are representative of three separate experiments with three rats pooled in each treatment group. *P<0.001 versus Nx+S and Nx+T; P<0.01 versus HR+T in fraction 7. *P<0.01 versus Nx+S and Nx+T; P<0.05 versus HR+T in fraction 8.
Similar articles
- Occludin oligomeric assemblies at tight junctions of the blood-brain barrier are altered by hypoxia and reoxygenation stress.
McCaffrey G, Willis CL, Staatz WD, Nametz N, Quigley CA, Hom S, Lochhead JJ, Davis TP. McCaffrey G, et al. J Neurochem. 2009 Jul;110(1):58-71. doi: 10.1111/j.1471-4159.2009.06113.x. Epub 2009 May 15. J Neurochem. 2009. PMID: 19457074 Free PMC article. - Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo.
McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, Davis TP. McCaffrey G, et al. J Neurochem. 2007 Dec;103(6):2540-55. doi: 10.1111/j.1471-4159.2007.04943.x. J Neurochem. 2007. PMID: 17931362 - Tempol modulates changes in xenobiotic permeability and occludin oligomeric assemblies at the blood-brain barrier during inflammatory pain.
Lochhead JJ, McCaffrey G, Sanchez-Covarrubias L, Finch JD, Demarco KM, Quigley CE, Davis TP, Ronaldson PT. Lochhead JJ, et al. Am J Physiol Heart Circ Physiol. 2012 Feb 1;302(3):H582-93. doi: 10.1152/ajpheart.00889.2011. Epub 2011 Nov 11. Am J Physiol Heart Circ Physiol. 2012. PMID: 22081706 Free PMC article. - The blood-brain barrier/neurovascular unit in health and disease.
Hawkins BT, Davis TP. Hawkins BT, et al. Pharmacol Rev. 2005 Jun;57(2):173-85. doi: 10.1124/pr.57.2.4. Pharmacol Rev. 2005. PMID: 15914466 Review. - Critical role of actin in modulating BBB permeability.
Lai CH, Kuo KH, Leo JM. Lai CH, et al. Brain Res Brain Res Rev. 2005 Dec 1;50(1):7-13. doi: 10.1016/j.brainresrev.2005.03.007. Brain Res Brain Res Rev. 2005. PMID: 16291072 Review.
Cited by
- Targeting blood-brain barrier changes during inflammatory pain: an opportunity for optimizing CNS drug delivery.
Ronaldson PT, Davis TP. Ronaldson PT, et al. Ther Deliv. 2011 Aug;2(8):1015-41. doi: 10.4155/tde.11.67. Ther Deliv. 2011. PMID: 22468221 Free PMC article. Review. - Physiology, pathophysiology and (mal)adaptations to chronic apnoeic training: a state-of-the-art review.
Elia A, Gennser M, Harlow PS, Lees MJ. Elia A, et al. Eur J Appl Physiol. 2021 Jun;121(6):1543-1566. doi: 10.1007/s00421-021-04664-x. Epub 2021 Mar 31. Eur J Appl Physiol. 2021. PMID: 33791844 Free PMC article. Review. - Postoperative Delirium in Neurosurgical Patients: Recent Insights into the Pathogenesis.
Xu Y, Ma Q, Du H, Yang C, Lin G. Xu Y, et al. Brain Sci. 2022 Oct 9;12(10):1371. doi: 10.3390/brainsci12101371. Brain Sci. 2022. PMID: 36291305 Free PMC article. Review. - Resolving Geroplasticity to the Balance of Rejuvenins and Geriatrins.
Tabibzadeh S. Tabibzadeh S. Aging Dis. 2022 Dec 1;13(6):1664-1714. doi: 10.14336/AD.2022.0414. eCollection 2022 Dec 1. Aging Dis. 2022. PMID: 36465174 Free PMC article. - Shielding Effect of Ryanodine Receptor Modulator in Rat Model of Autism.
Kumar H, Kulkarni GT, Diwan V, Sharma B. Kumar H, et al. Basic Clin Neurosci. 2023 Mar-Apr;14(2):247-261. doi: 10.32598/bcn.2021.2966.1. Epub 2023 Mar 1. Basic Clin Neurosci. 2023. PMID: 38107532 Free PMC article.
References
- Amadio M, Scapagnini G, Laforenza U, Intrieri M, Romeo L, Govoni S, Pascale A. -transcriptional regulation of HSP 70 expression followingoxidative stress in SH-SY5Y cells: the potential involvement of the RNA-binding protein HuR. Curr Pharm Des. 2008;14:2651–2658. - PubMed
- Augusto O, Trindade DF, Linares E, Vaz SM. Cyclid nitroxides inhibit the toxicity of nitric oxide-derived oxidants: mechanisms and implications. An Acad Bras Cienc. 2008;80:179–189. - PubMed
- Balda MS, Flores-Maldonado C, Cereijido M, Matter K. Multiple domains of occludin are involved in the regulation of paracellular permeability. J Cell Biochem. 2000;78:85–96. - PubMed
- Bhattacharjee AK, Nagashima T, Kondoh T, Tamaki N. Quantification of early blood-brain barrier disruption by in situ brain perfusion technique. Brain Res Brain Res Protoc. 2001;8:126–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources