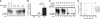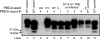Coinfecting prion strains compete for a limiting cellular resource - PubMed (original) (raw)
Coinfecting prion strains compete for a limiting cellular resource
Ronald A Shikiya et al. J Virol. 2010 Jun.
Abstract
Prion strain interference can influence the emergence of a dominant strain from a mixture; however, the mechanisms underlying prion strain interference are poorly understood. In our model of strain interference, inoculation of the sciatic nerve with the drowsy (DY) strain of the transmissible mink encephalopathy (TME) agent prior to superinfection with the hyper (HY) strain of TME can completely block HY TME from causing disease. We show here that the deposition of PrP(Sc), in the absence of neuronal loss or spongiform change, in the central nervous system corresponds with the ability of DY TME to block HY TME infection. This suggests that DY TME agent-induced damage is not responsible for strain interference but rather prions compete for a cellular resource. We show that protein misfolding cyclic amplification (PMCA) of DY and HY TME maintains the strain-specific properties of PrP(Sc) and replicates infectious agent and that DY TME can interfere, or completely block, the emergence of HY TME. DY PrP(Sc) does not convert all of the available PrP(C) to PrP(Sc) in PMCA, suggesting the mechanism of prion strain interference is due to the sequestering of PrP(C) and/or other cellular components required for prion conversion. The emergence of HY TME in PMCA was controlled by the initial ratio of the TME agents. A higher ratio of DY to HY TME agent is required for complete blockage of HY TME in PMCA compared to several previous in vivo studies, suggesting that HY TME persists in animals coinfected with the two strains. This was confirmed by PMCA detection of HY PrP(Sc) in animals where DY TME had completely blocked HY TME from causing disease.
Figures
FIG. 1.
The DY TME agent does not significantly inhibit retrograde transport or cause death of VMNs. Lumbar spinal cord sections from hamsters that were injected in the sciatic nerve with dextran conjugated to Alexa Fluor 568 at 120 days after sciatic nerve inoculation with either an uninfected brain homogenate (A) or DY TME agent (B). Insets in panels A and B depict dextran-positive ventral motor neurons. (C) The number of VMNs containing dextran in the lumbar spinal cord from the DY TME agent infected did not differ significantly (P > 0.05) compared to the negative control animals. The number of VMNs in Nissl-stained sections of lumbar spinal cord collected from hamsters at 120 days after sciatic nerve inoculation with the DY TME agent contralateral (D) or ipsilateral (E) to the side of inoculation did not significantly (P > 0.05) differ (F). The yellow regions in the schematic insets depict the location of the spinal cord that were imaged. Scale bar, 100 μm.
FIG. 2.
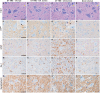
PrPSc deposition is present in the absence of spongiform degeneration, astrocytosis, microgliosis, and synaptic loss in the lumbar spinal cord at 134 days after DY TME agent inoculation. Hamsters were inoculated in the sciatic nerve with either the DY TME agent or uninfected (Mock) brain homogenate and lumbar spinal cord was collected at 134 days postinfection or at clinical disease. Lumbar spinal cord sections were stained either with hematoxylin and eosin (H&E) or immunohistochemistry was performed using antibodies directed against PrPSc, glial fibrillary acidic protein (GFAP), ionized calcium-binding adaptor molecule 1 (Iba-1) or synaptophysin. The yellow regions in the schematic insets depict the location of the brain that were imaged for each column. Arrows indicate PrPSc deposits in the panel E and G insets. Scale bar, 50 μm.
FIG. 3.
Strain-specific characteristics of HY and DY PrPSc are recapitulated by PMCA. (A) PMCA reactions were seeded with uninfected (UN), DY TME-infected (DY), or HY TME-infected brain homogenates (HY) and one round of PMCA resulted in detection of PK digestion-resistant PrPSc in the DY TME- and HY TME-seeded reactions but not in the negative control uninfected seeded reactions. (B) HY PrPSc accumulated to a higher abundance compared to DY PrPSc, suggesting that HY TME amplifies more efficiently than DY PrPSc. (C) Western blot analysis of PK-digested brain homogenates from HY TME (HY), DY TME (DY), or uninfected hamster (UN) before (lanes 1 to 3, 7, and 8) or after PMCA (lanes 4 to 6, 9, and 10) treated without (1-6) or with (7-10) PNGase indicates that the strain-specific migration of HY TME and DY TME PrPSc is maintained after PMCA. The migration of the 19- and 21-kDa unglycosylated PrPSc polypeptide is indicated on the left of the panel. (D) Survival of Syrian hamsters after i.c. inoculation of HY TME agent (n = 5, ⧫), DY TME agent (n = 5, ⋄), or the tenth serial PMCA round of HY TME (n = 5, ▪)-, DY TME (n = 4, □)-, or mock (n = 5, •)-seeded reactions.
FIG. 4.
Protein misfolding cyclic amplification strain interference. Known amounts of HY TME-infected and DY TME-infected brain homogenate were combined and added to PMCA reactions, and 10 rounds of serial PMCA were performed. (A) After four rounds of serial PMCA, Western blot analysis determined that PrPSc migration was consistent with HY PrPSc when the initial ratio of DY TME to HY TME brain homogenate ranged from 1:1 to 1:1,000, respectively (lanes 3 to 6) and was consistent with DY PrPSc when the ratio of DY TME to HY TME brain homogenate was greater than 1:10,000 (lanes 7 to 11). (B) When the ratio of DY to HY TME brain homogenate was 1:10,000, the migration of PrPSc was consistent with DY PrPSc during the first eight rounds of serial PMCA (lanes 3 to 8) but then changed to a migration pattern consistent with HY PrPSc during rounds 9 and 10 (lanes 11 to 12).
FIG. 5.
Persistence of HY TME in animals coinfected with the DY TME and HY TME agents. Four rounds of serial PMCA was performed on brain homogenate from uninfected (UN) hamsters, hamsters infected with either the HY TME agent (HY) or the DY TME agent (DY), or hamsters infected under conditions where the DY TME agent completely blocks the HY TME agent from causing disease. Western blot analysis of PK-digested sample from the uninfected negative control PMCA indicated that it did not amplify PrPSc (lane 3). The HY TME and DY TME positive control PMCA reactions amplified PrPSc that maintained the 21- and 19-kDa strain-specific PrPSc migration pattern (lanes 4 and 5, respectively). In the group of superinfected hamsters, one round of PMCA results in a 19-kDa PrPSc migration pattern consistent with DY PrPSc (compare lanes 5 and 6). After four rounds of serial PMCA, the PrPSc migration pattern is consistent with HY PrPSc (compare lanes 9 to 10). The migration of the 19- and 21-kDa unglycosylated PrPSc polypeptide is indicated on the left of the panel.
Similar articles
- Prion interference is due to a reduction in strain-specific PrPSc levels.
Bartz JC, Kramer ML, Sheehan MH, Hutter JA, Ayers JI, Bessen RA, Kincaid AE. Bartz JC, et al. J Virol. 2007 Jan;81(2):689-97. doi: 10.1128/JVI.01751-06. Epub 2006 Nov 1. J Virol. 2007. PMID: 17079313 Free PMC article. - Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy.
Bartz JC, Bessen RA, McKenzie D, Marsh RF, Aiken JM. Bartz JC, et al. J Virol. 2000 Jun;74(12):5542-7. doi: 10.1128/jvi.74.12.5542-5547.2000. J Virol. 2000. PMID: 10823860 Free PMC article. - Prion interference with multiple prion isolates.
Schutt CR, Bartz JC. Schutt CR, et al. Prion. 2008 Apr-Jun;2(2):61-3. doi: 10.4161/pri.2.2.6806. Epub 2008 Apr 18. Prion. 2008. PMID: 19098442 Free PMC article. Review. - Prion disease: a deadly disease for protein misfolding.
Chakraborty C, Nandi S, Jana S. Chakraborty C, et al. Curr Pharm Biotechnol. 2005 Apr;6(2):167-77. doi: 10.2174/1389201053642321. Curr Pharm Biotechnol. 2005. PMID: 15853695 Review.
Cited by
- Emergence of two prion subtypes in ovine PrP transgenic mice infected with human MM2-cortical Creutzfeldt-Jakob disease prions.
Chapuis J, Moudjou M, Reine F, Herzog L, Jaumain E, Chapuis C, Quadrio I, Boulliat J, Perret-Liaudet A, Dron M, Laude H, Rezaei H, Béringue V. Chapuis J, et al. Acta Neuropathol Commun. 2016 Feb 5;4:10. doi: 10.1186/s40478-016-0284-9. Acta Neuropathol Commun. 2016. PMID: 26847207 Free PMC article. - Mitigation of prion infectivity and conversion capacity by a simulated natural process--repeated cycles of drying and wetting.
Yuan Q, Eckland T, Telling G, Bartz J, Bartelt-Hunt S. Yuan Q, et al. PLoS Pathog. 2015 Feb 9;11(2):e1004638. doi: 10.1371/journal.ppat.1004638. eCollection 2015 Feb. PLoS Pathog. 2015. PMID: 25665187 Free PMC article. - Isolation of novel synthetic prion strains by amplification in transgenic mice coexpressing wild-type and anchorless prion proteins.
Raymond GJ, Race B, Hollister JR, Offerdahl DK, Moore RA, Kodali R, Raymond LD, Hughson AG, Rosenke R, Long D, Dorward DW, Baron GS. Raymond GJ, et al. J Virol. 2012 Nov;86(21):11763-78. doi: 10.1128/JVI.01353-12. Epub 2012 Aug 22. J Virol. 2012. PMID: 22915801 Free PMC article. - Development of a methodology for large-scale production of prions for biological and structural studies.
Concha-Marambio L, Wang F, Armijo E, Gorski D, Ramirez F, Scowcroft A, Pritzkow S, Soto C. Concha-Marambio L, et al. Front Mol Biosci. 2023 Aug 10;10:1184029. doi: 10.3389/fmolb.2023.1184029. eCollection 2023. Front Mol Biosci. 2023. PMID: 37635939 Free PMC article. - Prion Strains and Transmission Barrier Phenomena.
Igel-Egalon A, Béringue V, Rezaei H, Sibille P. Igel-Egalon A, et al. Pathogens. 2018 Jan 1;7(1):5. doi: 10.3390/pathogens7010005. Pathogens. 2018. PMID: 29301257 Free PMC article. Review.
References
- Alais, S., S. Simoes, D. Baas, S. Lehmann, G. Raposo, J. L. Darlix, and P. Leblanc. 2008. Mouse neuroblastoma cells release prion infectivity associated with exosomal vesicles. Biol. Cell 100:603-615. - PubMed
- Bartz, J. C., J. M. Aiken, and R. A. Bessen. 2004. Delay in onset of prion disease for the HY strain of transmissible mink encephalopathy as a result of prior peripheral inoculation with the replication-deficient DY strain. J. Gen. Virol. 85:265-273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G20RR024001/RR/NCRR NIH HHS/United States
- G20 RR024001/RR/NCRR NIH HHS/United States
- C06 RR17417-01/RR/NCRR NIH HHS/United States
- R01 NS052609/NS/NINDS NIH HHS/United States
- C06 RR017417/RR/NCRR NIH HHS/United States
- P20 RR0115635-6/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials