Impaired ganglioside metabolism in Huntington's disease and neuroprotective role of GM1 - PubMed (original) (raw)
Comparative Study
Impaired ganglioside metabolism in Huntington's disease and neuroprotective role of GM1
Vittorio Maglione et al. J Neurosci. 2010.
Abstract
Huntington's disease (HD) is a neurodegenerative disorder caused by the expansion of a polyglutamine stretch in the protein huntingtin (Htt). HD neurons are dysfunctional at multiple levels and have increased susceptibility to stress and apoptotic stimuli. We have discovered that synthesis of the ganglioside GM1 is reduced in fibroblasts from HD patients and in cell and animal models of HD, and that decreased GM1 levels contribute to heighten HD cell susceptibility to apoptosis. The apoptotic susceptibility is recapitulated through inhibition of ganglioside synthesis in wild-type striatal cells, suggesting that decreased GM1 levels might be one of the key events leading to HD pathogenesis and progression. Administration of GM1 restores ganglioside levels in HD cells and promotes activation of AKT and phosphorylation of mutant Htt, leading to decreased mutant Htt toxicity and increased survival of HD cells. Our data identify GM1 as a potential treatment for HD.
Figures
Figure 1.
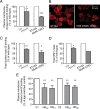
Levels of the ganglioside GM1 are reduced in HD cells. A, Quantitation of plasma membrane GM1 by Alexa488-conjugated cholera toxin B labeling and FACS analysis. Data represent the mean ± SD of three to five independent experiments, each performed in triplicate. B, Confocal microscopy images of parental (ST14A) and mHtt-expressing cells (N548-120Q) labeled with Alexa594-conjugated cholera toxin B at 4°C to visualize plasma membrane GM1. Images are three-dimensional reconstructions of confocal Z-stacks. C, Total (plasma membrane plus intracellular) GM1 measured by cholera toxin B labeling of fixed and permeabilized cells. Data are the mean ± SD of three experiments. D, Total GM1 measured in cell gangliosides extracts separated and visualized on TLC as described in Materials and Methods. Because of the large number of cells required, this confirmatory experiment was performed only once in ST14A and N548-120Q cells, and therefore SDs were not included in the graph. E, Plasma membrane GM1 levels in human fibroblasts from HD patients and normal subjects. HD fibroblasts were compared with age-matched controls at the same passage number (C1 and C2). Subscripts represent the number of CAG repeats in the HD gene of the affected individuals. Additional information on human fibroblast lines is provided in supplemental Table 1 (available at
as supplemental material). Data are the mean ± SD of three experiments performed on cells at three different passages in culture. 7/7, ST_Hdh_7/7; 111/111, ST_Hdh_111/111; *p < 0.05; **p < 0.001; ***p < 0.0001.
Figure 2.
Expression of B3galt4 (GM1/GD1b synthase) is downregulated in striatal cell models of HD and in fibroblasts from HD patients. A, B, Gene expression analysis by quantitative PCR in striatal knock-in cells, rat striatal parental (ST14A) and mHtt-expressing cells (N548-120Q) (A), and human fibroblasts (B). Data were normalized to cyclophilin A expression. HD fibroblasts were compared with age-matched controls at the same passage number (C1 and C2). Subscripts represent the number of CAG repeats in the HD gene of the affected individuals. Additional information on the human fibroblast lines is provided in supplemental Table 1 (available at
as supplemental material). Data are the mean ± SD of two to four independent experiments. 7/7, ST_Hdh_7/7; 111/111, ST_Hdh_111/111; *p < 0.05; **p < 0.001; ***p < 0.0001.
Figure 3.
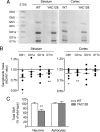
GM1 is reduced in striatum, cortex, and neurons of YAC128 mice. A, Representative TLC analysis of striatal and cortical gangliosides. Ganglioside standards (STDS) are indicated. B, Densitometric analysis of individual gangliosides extracted from brain regions of six YAC128 mice, separated by TLC and compared with six wild-type littermates. Each dot represents one individual YAC128 mouse. Data are expressed as ratios of HD to wild-type control. C, Total GM1 levels in primary cultures of neurons and astrocytes were measured by dot-blotting with HRP-conjugated cholera toxin B. Individual neural cultures from 12 YAC128 newborn mice and 12 wild-type littermates were analyzed. Astrocyte cultures from 16 YAC128 and 18 wild-type littermates were used. Data are expressed as the mean percentage of the average of wild-type values ± SD. *p < 0.05; **p < 0.001.
Figure 4.
Specific enzymes of the ganglioside biosynthetic pathway are downregulated in striatum and cortex of YAC128 mice. A, Simplified scheme of the ganglioside biosynthetic pathway. Critical enzymes in the pathway are indicated in red. Gangliosides of the asialo-series and of the c-series are less abundant and were omitted to improve clarity. B, Analysis of gene expression in YAC128 mice by real-time PCR. Each dot represents an individual animal. From five to six animals were used in the analysis. Lines indicate the mean value in each group. Data are expressed as ratios of HD to wild type. *p < 0.05; **p < 0.001; ***p < 0.0001.
Figure 5.
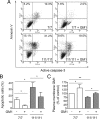
GM1 administration raises plasma membrane ganglioside levels and protects cells from apoptosis. Cells were incubated with or without 50 μ
m
GM1 and exposed to apoptotic conditions (serum deprivation at 39°C). A, Representative FACS profile of cells after 12 h incubation in apoptotic conditions. Cells were labeled with Annexin V and then stained for active caspase-3. FL1-H and FL2-H indicate fluorescence intensity for active caspase-3 and annexin V staining, respectively. Double-positive cells in the upper right quadrant are apoptotic cells. Annexin V-positive cells, but active caspase 3-negative cells (upper left quadrant) are early apoptotic cells. The number reported in each quadrant represents the percentage of cells in the quadrant. B, Quantitation of apoptotic cells by FACS analysis of annexin V binding. Data are the mean ± SD of four experiments, each performed in triplicate. C, Plasma membrane GM1 levels were measured after GM1 administration by analysis of cholera toxin B binding. Data are expressed as the percentage of control (wild type in basal conditions) and represent the mean ± SD of three experiments, each performed in triplicate. 7/7, ST_Hdh_7/7; 111/111, ST_Hdh_111/111; *p < 0.05; **p < 0.001.
Figure 6.
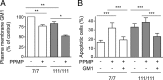
Inhibition of GM1 biosynthesis renders wild-type cells more susceptible to apoptosis. Cells were treated with 10 μ
m
PPMP, an inhibitor of the ganglioside biosynthetic pathway, for 3 d and then exposed to apoptotic conditions (serum deprivation at 39°C). A, Plasma membrane levels of GM1 were measured by cholera toxin B-binding and FACS analysis after incubation with PPMP. B, The percentage of apoptotic cells on incubation of the cells for 12 h in serum-free medium was measured by annexin V binding. Data are the mean ± SD of three experiments. 7/7, ST_Hdh_7/7; 111/111, ST_Hdh_111/111; *p < 0.05; **p < 0.001; ***p < 0.0001.
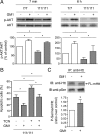
Figure 7.
Administration of GM1 results in AKT activation and increased mHtt phosphorylation. A, Akt phosphorylation was measured 7 min and 6 h after addition of GM1 in serum-free medium and incubation at 39°C to induce cell death. Analysis of phospho-AKT/AKT ratio, indicative of AKT activation, was performed by densitometric analysis of immunoblots in three independent experiments. In each experiment, the phospho-AKT/AKT ratio measured in wild-type untreated cells (control) was arbitrarily set to 100%, to which HD and GM1-treated samples were compared. Mean values ± SD and representative immunoblots are shown. B, Cells were preincubated for 2 h with 1 μ
m
TCN, an inhibitor of AKT activation, before GM1 administration and exposure to apoptotic stress (serum-free medium at 39°C). The percentage of apoptotic cells was measured by annexin V binding. Data are the mean ± SD of three experiments, each performed in triplicate. C, ST_Hdh_111/111 cells were incubated with or without GM1 for 5 min. Mutant Htt was immunoprecipitated and its phosphorylation state was assessed by immunoblotting with anti-pSer (phospho-Ser) antibody. A representative immunoblot is shown, along with the densitometric analysis performed on four independent experiments. In each experiment, the ratio p-Ser/mHtt measured in untreated cells was arbitrarily set to 1, and the ratio obtained for GM1-treated cells was compared with it. Statistical significance was determined by two-tailed t test. 7/7, ST_Hdh_7/7; 111/111, ST_Hdh_111/111; FL-mHtt, full-length mHtt; *p < 0.05.
Similar articles
- The cytoprotective role of GM1 ganglioside in Huntington disease cells.
Hart HS, Valentin MA, Peters ST, Holler SW, Wang H, Harmon AF, Holler LD. Hart HS, et al. Mol Biol Rep. 2022 Dec;49(12):12253-12258. doi: 10.1007/s11033-022-07830-2. Epub 2022 Sep 30. Mol Biol Rep. 2022. PMID: 36180805 Free PMC article. - CEP-1347 reduces mutant huntingtin-associated neurotoxicity and restores BDNF levels in R6/2 mice.
Apostol BL, Simmons DA, Zuccato C, Illes K, Pallos J, Casale M, Conforti P, Ramos C, Roarke M, Kathuria S, Cattaneo E, Marsh JL, Thompson LM. Apostol BL, et al. Mol Cell Neurosci. 2008 Sep;39(1):8-20. doi: 10.1016/j.mcn.2008.04.007. Epub 2008 Apr 24. Mol Cell Neurosci. 2008. PMID: 18602275 - PRMT5- mediated symmetric arginine dimethylation is attenuated by mutant huntingtin and is impaired in Huntington's disease (HD).
Ratovitski T, Arbez N, Stewart JC, Chighladze E, Ross CA. Ratovitski T, et al. Cell Cycle. 2015;14(11):1716-29. doi: 10.1080/15384101.2015.1033595. Cell Cycle. 2015. PMID: 25927346 Free PMC article. - Selective degeneration in YAC mouse models of Huntington disease.
Van Raamsdonk JM, Warby SC, Hayden MR. Van Raamsdonk JM, et al. Brain Res Bull. 2007 Apr 30;72(2-3):124-31. doi: 10.1016/j.brainresbull.2006.10.018. Epub 2006 Nov 16. Brain Res Bull. 2007. PMID: 17352936 Review. - Wild-type huntingtin plays a role in brain development and neuronal survival.
Reiner A, Dragatsis I, Zeitlin S, Goldowitz D. Reiner A, et al. Mol Neurobiol. 2003 Dec;28(3):259-76. doi: 10.1385/MN:28:3:259. Mol Neurobiol. 2003. PMID: 14709789 Review.
Cited by
- Deciphering mouse brain spatial diversity via glyco-lipidomic mapping.
Lee J, Yin D, Yun J, Kim M, Kim SW, Hwang H, Park JE, Lee B, Lee CJ, Shin HS, An HJ. Lee J, et al. Nat Commun. 2024 Oct 7;15(1):8689. doi: 10.1038/s41467-024-53032-8. Nat Commun. 2024. PMID: 39375371 Free PMC article. - Gangliosides as Therapeutic Targets for Neurodegenerative Diseases.
Inci OK, Basırlı H, Can M, Yanbul S, Seyrantepe V. Inci OK, et al. J Lipids. 2024 Apr 8;2024:4530255. doi: 10.1155/2024/4530255. eCollection 2024. J Lipids. 2024. PMID: 38623278 Free PMC article. Review. - Ganglioside-focused Glycan Array Reveals Abnormal Anti-GD1b Auto-antibody in Plasma of Preclinical Huntington's Disease.
Lin TW, Chang JK, Wu YR, Sun TH, Cheng YY, Ren CT, Pan MH, Wu JL, Chang KH, Yang HI, Chen CM, Wu CY, Chen YR. Lin TW, et al. Mol Neurobiol. 2023 Jul;60(7):3873-3882. doi: 10.1007/s12035-023-03307-w. Epub 2023 Mar 28. Mol Neurobiol. 2023. PMID: 36976478 - Age-dependent and regional heterogeneity in the long-chain base of A-series gangliosides observed in the rat brain using MALDI Imaging.
Caughlin S, Maheshwari S, Weishaupt N, Yeung KK, Cechetto DF, Whitehead SN. Caughlin S, et al. Sci Rep. 2017 Nov 23;7(1):16135. doi: 10.1038/s41598-017-16389-z. Sci Rep. 2017. PMID: 29170521 Free PMC article. - Glycosphingolipid-Protein Interaction in Signal Transduction.
Russo D, Parashuraman S, D'Angelo G. Russo D, et al. Int J Mol Sci. 2016 Oct 15;17(10):1732. doi: 10.3390/ijms17101732. Int J Mol Sci. 2016. PMID: 27754465 Free PMC article. Review.
References
- Abad-Rodriguez J, Robotti A. Regulation of axonal development by plasma membrane gangliosides. J Neurochem. 2007;103(Suppl 1):47–55. - PubMed
- Alter M. GM1 ganglioside for acute ischemic stroke. Trial design issues. Ann N Y Acad Sci. 1998;845:391–401. - PubMed
- Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, Hayward SD, Moran TH, Montell C, Ross CA, Snyder SH, Sawa A. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington's disease. Neuron. 2005;47:29–41. - PubMed
- Chiavegatto S, Sun J, Nelson RJ, Schnaar RL. A functional role for complex gangliosides: motor deficits in GM2/GD2 synthase knockout mice. Exp Neurol. 2000;166:227–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical