MicroRNA-210: a unique and pleiotropic hypoxamir - PubMed (original) (raw)
Review
. 2010 Mar 15;9(6):1072-83.
doi: 10.4161/cc.9.6.11006. Epub 2010 Mar 15.
Affiliations
- PMID: 20237418
- PMCID: PMC2912143
- DOI: 10.4161/cc.9.6.11006
Review
MicroRNA-210: a unique and pleiotropic hypoxamir
Stephen Y Chan et al. Cell Cycle. 2010.
Abstract
Inadequate oxygen availability or hypoxia induces a complex and still incompletely understood set of adaptations that influence cellular survival and function. Many of these adaptations are directly controlled by a master transcription factor, hypoxia inducible factor-alpha (HIF-α). In response to hypoxia, HIF-α levels increase and directly induce the transcription of > 100 genes, influencing functions ranging from metabolism, survival, proliferation, migration, to angiogenesis, among others. Recently, it has been demonstrated that a specific set of microRNA molecules are upregulated by hypoxia, which we denote here as "hypoxamirs." In particular, the HIF-responsive hypoxamir microRNA-210 (miR-210) is a unique microRNA that is evolutionarily conserved and ubiquitously expressed in hypoxic cell and tissue types. A number of direct targets of miR-210 have been identified by in silico, transcriptional, and biochemical methods, a subset of which have been extensively validated. As a result, miR-210 has been mechanistically linked to the control of a wide range of cellular responses known to influence normal developmental physiology as well as a number of hypoxia-dependent disease states, including tissue ischemia, inflammation, and tumorigenesis. Thus, reflecting the pleiotropic actions of HIF-α, miR-210 appears to function as a "master microRNA" relevant for the control of diverse functions in the hypoxic state.
Figures
Figure 1
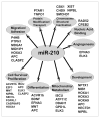
MiR-210 directly represses multiple transcripts associated with diverse cellular functions. To date, at least 35 unique transcripts (denoted by their official gene symbol) have been identified and further verified as direct targets of repression by miR-210. These transcripts are mechanistically linked to a wide array of fundamental cellular processes (denoted in the gray ovals) that are critical to hypoxic survival and adaptation of the mammalian cell. Here, verified targets of miR-210 are listed if previously found to be downregulated by miR-210, either through gain-of-function (forced expression of miR-210 during normoxia) or loss-of-function (inhibition of endogenous miR-210 during hypoxia) assays, coupled with either confirmation of specific recognition of the predicted target sequence by miR-210 (i.e., transactivation luciferase assays) or biochemical immunoprecipitation of the target transcript by miR-210-enriched AGO2/RISC. Notably, additional in silico-predicted transcripts have been further implicated as possible targets of miR-210 through either miR-210-dependent immunoprecipitation of AGO2/RISC or transcriptional/proteomic high-throughput analysis after manipulation of miR-210 expression. We expect that a significant percentage of these candidates will be confirmed as targets in the future, and as a result, the number of associated cellular processes controlled by this versatile hypoxamir will continue to expand.
Figure 2
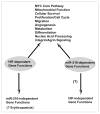
HIF-dependent gene pathways coincide with miR-210-dependent gene pathways. Pathways of gene regulation in endothelial cells by miR-210 as assessed by transcriptional and proteomic analyses intersect with a majority of well-characterized gene expression changes induced by HIF-1α during hypoxic exposure. While some HIF-dependent processes have not been definitively implicated in the actions of miR-210 (i.e., direct erythropoiesis), it is still possible that a subset of these actions may be linked to miR-210 in separate cell-specific or environmental contexts. Furthermore, while HIF-1α appears to play a predominant role in the induction of miR-210, baseline, endogenous levels of miR-210 are higher in certain cell types and may carry some biological activity during normoxia. Thus, HIF-independent effects of miR-210 are possible and await further clarification.
Figure 3
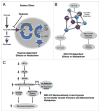
MiR-210 inhibits mitochondrial metabolism via repression of ISCU1/2 and iron-sulfur cluster-dependent function. (A) Louis Pasteur first described a fundamental cellular response to hypoxia, resulting in a metabolic shift from mitochondrial oxidative phosphorylation to glycolysis. The molecular mechanisms underlying this complex event have been incompletely characterized to date. (B) Iron-sulfur cluster assembly proteins ISCU1/2 are essential for iron-sulfur cluster biogenesis. These moieties are incorporated into a wide variety of proteins, many of which are involved in mitochondrial metabolism and aerobic energy production. While the function of iron-sulfur clusters has been more extensively studied in bacteria and yeast, less is known regarding their dynamic regulation and function in hypoxic mammalian cells. (C) MiR-210 regulates iron-sulfur cluster-dependent metabolic function during hypoxia via direct repression of ISCU1/2, leading to downregulate iron-sulfur cluster biogenesis and iron-sulfur-dependent metabolic enzyme activity. In doing so, miR-210 disrupts mitochondrial respiration and potentially other iron-sulfur cluster (ISC)-dependent functions such as iron metabolism, among others. As a result, miR-210 modulates a unique constellation of essential metabolic functions that predominates in the Pasteur effect, and influences cellular adaptation to hypoxia in the mammalian cell.
Figure 4
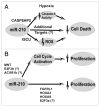
Complex control of cellular survival and proliferation by miR-210. (A) MiR-210 increases cell survival during hypoxia by modulating multiple direct targets involved in apoptosis and cell death. By repressing CASP8AP2 and directly modulating caspase-8 activity, miR-210 improves survival of mesenchymal stem cells after engraftment into infarcted cardiac tissue. Furthermore, by repressing ISCU1/2, miR-210 appears to decrease ROS levels and thereby improve survival of human pulmonary vascular endothelial cells exposed to hypoxia. There likely exist additional direct targets of miR-210 that further contribute to its pro-survival and adaptive functions during hypoxic stress. (B) MiR-210 may induce cell type-specific control of proliferation. In certain transformed cells, inhibition of direct target MNT and perhaps E2F3b and ACVR1b by miR-210 leads to an activation of G1/S cell cycle progression and increased cellular proliferation. However, in other transformed and tumor cells, inhibition of FGFRL1, HOXA3, and, perhaps, HOXA9 and E2F3a appear to reduce cellular proliferation. It is unclear how to reconcile these seemingly paradoxical findings. However, it is reasonable to hypothesize that a given cell type or basal milieu may carry different subsets of the total complement of targets of miR-210 and, thus, differentially respond to the control of cellular proliferation based on those distinct targets.
Similar articles
- The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1α expression in human endothelial cells through a negative feedback loop.
Bartoszewska S, Kochan K, Piotrowski A, Kamysz W, Ochocka RJ, Collawn JF, Bartoszewski R. Bartoszewska S, et al. FASEB J. 2015 Apr;29(4):1467-79. doi: 10.1096/fj.14-267054. Epub 2014 Dec 30. FASEB J. 2015. PMID: 25550463 Free PMC article. - Hypoxia: a master regulator of microRNA biogenesis and activity.
Nallamshetty S, Chan SY, Loscalzo J. Nallamshetty S, et al. Free Radic Biol Med. 2013 Sep;64:20-30. doi: 10.1016/j.freeradbiomed.2013.05.022. Epub 2013 May 24. Free Radic Biol Med. 2013. PMID: 23712003 Free PMC article. Review. - Enhanced ROBO4 is mediated by up-regulation of HIF-1α/SP1 or reduction in miR-125b-5p/miR-146a-5p in diabetic retinopathy.
Gong Q, Xie J, Li Y, Liu Y, Su G. Gong Q, et al. J Cell Mol Med. 2019 Jul;23(7):4723-4737. doi: 10.1111/jcmm.14369. Epub 2019 May 15. J Cell Mol Med. 2019. PMID: 31094072 Free PMC article. - Hypoxia-inducible miR-196a modulates glioblastoma cell proliferation and migration through complex regulation of NRAS.
Takkar S, Sharma V, Ghosh S, Suri A, Sarkar C, Kulshreshtha R. Takkar S, et al. Cell Oncol (Dordr). 2021 Apr;44(2):433-451. doi: 10.1007/s13402-020-00580-y. Epub 2021 Jan 19. Cell Oncol (Dordr). 2021. PMID: 33469841 - Impact of MicroRNAs in the Cellular Response to Hypoxia.
Bertero T, Rezzonico R, Pottier N, Mari B. Bertero T, et al. Int Rev Cell Mol Biol. 2017;333:91-158. doi: 10.1016/bs.ircmb.2017.03.006. Epub 2017 Apr 18. Int Rev Cell Mol Biol. 2017. PMID: 28729029 Review.
Cited by
- miR-roring Changes in Blood: miR-210 Reflects Hypoxic Disease Dynamics in Obstructive Sleep Apnea.
Kirillova A, Chan SY. Kirillova A, et al. Am J Respir Crit Care Med. 2023 Feb 1;207(3):240-242. doi: 10.1164/rccm.202209-1709ED. Am J Respir Crit Care Med. 2023. PMID: 36201758 Free PMC article. No abstract available. - Genetic and hypoxic alterations of the microRNA-210-ISCU1/2 axis promote iron-sulfur deficiency and pulmonary hypertension.
White K, Lu Y, Annis S, Hale AE, Chau BN, Dahlman JE, Hemann C, Opotowsky AR, Vargas SO, Rosas I, Perrella MA, Osorio JC, Haley KJ, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Khan OF, Bader A, Gochuico BR, Matar M, Polach K, Johannessen NM, Prosser HM, Anderson DG, Langer R, Zweier JL, Bindoff LA, Systrom D, Waxman AB, Jin RC, Chan SY. White K, et al. EMBO Mol Med. 2015 Jun;7(6):695-713. doi: 10.15252/emmm.201404511. EMBO Mol Med. 2015. PMID: 25825391 Free PMC article. - Up-Regulation of microRNA-210 is Associated with Spermatogenesis by Targeting IGF2 in Male Infertility.
Tang D, Huang Y, Liu W, Zhang X. Tang D, et al. Med Sci Monit. 2016 Aug 18;22:2905-10. doi: 10.12659/msm.897340. Med Sci Monit. 2016. PMID: 27535712 Free PMC article. - MicroRNA-687 Induced by Hypoxia-Inducible Factor-1 Targets Phosphatase and Tensin Homolog in Renal Ischemia-Reperfusion Injury.
Bhatt K, Wei Q, Pabla N, Dong G, Mi QS, Liang M, Mei C, Dong Z. Bhatt K, et al. J Am Soc Nephrol. 2015 Jul;26(7):1588-96. doi: 10.1681/ASN.2014050463. Epub 2015 Jan 13. J Am Soc Nephrol. 2015. PMID: 25587068 Free PMC article. - Emerging molecular targets for brain repair after stroke.
Krupinski J, Slevin M. Krupinski J, et al. Stroke Res Treat. 2013;2013:473416. doi: 10.1155/2013/473416. Epub 2013 Jan 13. Stroke Res Treat. 2013. PMID: 23365789 Free PMC article.
References
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. - PubMed
- Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–7. - PubMed
- Adams JM, Difazio LT, Rolandelli RH, Lujan JJ, Hasko G, Csoka B, et al. HIF-1: a key mediator in hypoxia. Acta Physiol Hung. 2009;96:19–28. - PubMed
- Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene upregulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005;65:3299–306. - PubMed
- Maynard M, Evans A, Hosomi T, Hara S, Jewett M, Ohh M. Human HIF-3alpha4 is a dominant-negative regulator of HIF-1 and is downregulated in renal cell carcinoma. FASEB J. 2005;19:1396–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 HV028178/HV/NHLBI NIH HHS/United States
- U54 HL070819/HL/NHLBI NIH HHS/United States
- R01 HL061795/HL/NHLBI NIH HHS/United States
- K08 HL096834/HL/NHLBI NIH HHS/United States
- N01 HV028178/HL/NHLBI NIH HHS/United States
- R37 HL061795/HL/NHLBI NIH HHS/United States
- U54 HL 70819/HL/NHLBI NIH HHS/United States
- HL 61795/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials