Modelling the molecular mechanisms of synaptic plasticity using systems biology approaches - PubMed (original) (raw)
Review
Modelling the molecular mechanisms of synaptic plasticity using systems biology approaches
Jeanette Hellgren Kotaleski et al. Nat Rev Neurosci. 2010 Apr.
Abstract
Synaptic plasticity is thought to underlie learning and memory, but the complexity of the interactions between the ion channels, enzymes and genes that are involved in synaptic plasticity impedes a deep understanding of this phenomenon. Computer modelling has been used to investigate the information processing that is performed by the signalling pathways involved in synaptic plasticity in principal neurons of the hippocampus, striatum and cerebellum. In the past few years, new software developments that combine computational neuroscience techniques with systems biology techniques have allowed large-scale, kinetic models of the molecular mechanisms underlying long-term potentiation and long-term depression. We highlight important advancements produced by these quantitative modelling efforts and introduce promising approaches that use advancements in live-cell imaging.
Figures
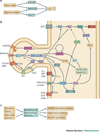
Display 1: Figure 1. Signaling pathways underlying synaptic plasticity
A. Presynaptic glutamate release and depolarization of the postsynaptic neuron lead to calcium elevation in the postsynaptic cell. Glutamate is required for activation of NMDA receptors and metabotropic glutamate receptors, and depolarization is required for activation of NMDA receptors and voltage-dependent calcium channels. The particular mechanism employed depends on the cell type. B. Signaling pathways leading to kinase activation and AMPAR phosphorylation. Only a subset of the known pathways is illustrated, and not all pathways are involved in all neurons. Calcium activates CaMKII, which phosphoryolates the AMPA receptor GluR1 subunit, leading to increased numbers of AMPA receptors. CaMKII can be persistently activated by autophosphorylation, , which occurs when two adjacent subunits are bound to calcium-calmodulin. This persistently active form of CaMKII is most strongly implicated in hippocampal LTP. Dopamine (D1) and β-adrenergic receptors, , coupled to GS or Golf, contribute to LTP by activating adenylyl cyclase, while other dopamine (D2) receptors and muscarinic acetylcholine (M2, M4) receptors inhibit adenylyl cyclase. The cAMP produced by adenylyl cyclase activates PKA which subsequently phosphorylates AMPA receptor GluR1 subunits and either DARPP32 (dopamine and cAMP regulated phosphoprotein of 32 kDa) or inhibitor-1, , which decrease phosphatase activity to allow persistence or enhancement of phosphorylation of AMPA receptors. Some types of muscarinic acetylcholine (M1) receptors and metabotropic glutamate receptors are coupled to phospholipase C (PLC), which produces diacylglycerol (DAG) and inositol triphosphate. Typical forms of PKC are activated by binding to both calcium and DAG. ERK is activated by a pathway involving tyrosine kinase receptors via the Ras-Raf-MEK pathway, and is necessary for the gene transcription and protein translation that underlies persistent forms of synaptic plasticity. In addition, ERK can be indirectly activated by PKC, EPAC, calcium and PKA. C. For late-phase LTP and memory storage, a combination of synaptic inputs and neuronal activity leads to AMPA receptor phosphorylation, gene transcription, and protein translation.
Display 2: Figure 2
A. Examples of common structures found in cell signaling networks that have been identified using graph theory. A1. A “Bow-tie” structure: signals from many receptors converge onto a few cytosolic targets which in turn regulate many transcription factors; A2. The “bifan” motif : two input nodes directly cross-regulate target nodes; A3. Negative feedback loops that include receptors are more often observed close to the membrane, and positive feedback loops (often highly nested) are more commonly found a few steps downstream from receptors. B. Dynamics in minimal pathway components in cell signaling networks. B1. Positive feedback loop. The feedback results in a larger response than if no feedback were present. The interaction between voltage and L type or non-inactivating inward calcium channels show this type of positive feedback. In some cases positive feedback might result in bistability; B2. Negative feedback loop with no (or in practical cases very little) delay. The feedback results in a smaller response than if no feedback were present. Non-inactivating outward K channels exhibit negative feedback; B3. Negative feedback loop with delay. The delayed feedback reduces the response after an initial transient response. Oscillations can occur in response to sustained input. C. A generic MAPK pathway. C1. A single phosphorylation-dephosphorylation cycle is circled in green. The MAPK cascade consists of three layers of such cycles, and the two lower layers have dual phosphorylation sites. C2. Phosphorylation-dephosphorylation cycles can in certain conditions show “ultrasensitivity” and behave as threshold devices. The layered cascade, with dual phosphorylation sites, can sustain bistability as well. Bistability requires hysterisis: the response to an increasing input differs from the response to a decreasing input. In contrast, ultrasensitivity exhibits a similar steep increase in output with small changes in input, but the threshold is identical for increasing and decreasing inputs.
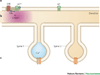
Display 3: Figure 3
Spatial representation of a dendrite plus multiple dendritic spines are required to address input specificity and microdomains. A. G proteins diffuse laterally within the membrane to activate adenylyl cyclase. Limited mobility, coupled with RGS activity keeps cyclase activation (AC*) confined to a region near the receptor, whereas AC further away remains inactive. B. cAMP diffuses rapidly within the cytosol; thus, a gradient of cAMP is less steep than that of active cyclase, unless mechanisms to maintain the gradient are present, such as localized PDEs. C. Synaptic activation of spine 1, but not spine 2, results in calcium elevation in spine 1, which remains limited to this spine by buffers and pumps. Otherwise, diffusion would carry the calcium message to spine 2, resulting in reduced synaptic specificity.
Display 4: Figure 4
Spatial stochastic simulations using NeuroRD evaluate the role of anchoring proteins and microdomains in synaptic plasticity of hippocampal area CA1 (Kim, Chay, Blackwell; personal communication). A. NeuroRD is software for simulating stochastic reaction-diffusion systems in complex 3-D morphologies on a mesoscopic scale. The software merges a mesoscopic diffusion algorithm with elements of the tau-leap reaction algorithm. The dendrite is subdivided into submembrane region and cytosolic region, and each region is further subdivided into voxels. Similarly, the spine is subdivided into head and neck voxels. Probability of reaction and diffusion are calculated from the voxel geometry, and either diffusion constant or reaction constant. Given the reaction and diffusion probabilities, each timestep involves generating the numbers of particles diffusing across each possible boundary, the number of reactions occurring, and updating the numbers of particles of each type in each voxel accordingly. The molecules and reactions in the system include the DaR1 and calcium activated pathways illustrated in Figure 1B, but do not include MAPK pathways, Gαi or Gαq coupled pathways. In both cases Da1R, G protein, AC1/8 are localized in the spine. In one condition, PKA is co-localized with AC in the spine (left), in the other condition, PKA is anchored to the dendrite submembrane. B. In both conditions, there is a calcium gradient from spine to dendrite. C. AC produces cAMP in the spine head, which diffuses into the dendrite, creating a gradient of cAMP. D. The gradient of cAMP leads to higher PKA activation when PKA is co-localized in the spine head. E. The difference in PKA activation propagates downstream to yield greater phosphorylation of Inhibitor1 or PP1 when PKA is in the spine.
Display 5: Figure 5
Computational neuroscience in the future will A. integrate systems biology approaches to modeling the signaling pathways underlying synaptic plasticity with computational neuroscience approaches to modeling electrical activity in the neuron. Both ionotropic and metabotropic receptors are activated by simulated synaptic inputs resulting from ongoing network activity. Membrane depolarization produces calcium influx through voltage dependent calcium channels, and in turn, calcium dependent potassium channels modify membrane potentiation. Both calcium and receptor activated signaling pathways activate kinases and phosphotases which may modify channel properties. B. Networks of such model neurons are required to understand the development of plasticity in response to realistic neuronal firing patterns, and to understand how the plasticity in turn modifies network activity. Thus, multiple copies of the neuron model of electrical and chemical events will be connected to each other with synapses. C. Neuroinformatics databases and tools will play a key role in facilitating development of large scale data-driven models, ranging from molecules to network connections. The information in the databases range from neuron morphology, to kinetics of signaling pathways and ionic channels, and will be used to create more realistic neuron models.
Similar articles
- Short-term depression and long-term plasticity together tune sensitive range of synaptic plasticity.
Deperrois N, Graupner M. Deperrois N, et al. PLoS Comput Biol. 2020 Sep 25;16(9):e1008265. doi: 10.1371/journal.pcbi.1008265. eCollection 2020 Sep. PLoS Comput Biol. 2020. PMID: 32976516 Free PMC article. - Synaptic signalling in cerebellar plasticity.
Evans GJ. Evans GJ. Biol Cell. 2007 Jul;99(7):363-78. doi: 10.1042/BC20070010. Biol Cell. 2007. PMID: 17567263 Review. - Molecular mechanisms underlying neuronal synaptic plasticity: systems biology meets computational neuroscience in the wilds of synaptic plasticity.
Blackwell KT, Jedrzejewska-Szmek J. Blackwell KT, et al. Wiley Interdiscip Rev Syst Biol Med. 2013 Nov-Dec;5(6):717-31. doi: 10.1002/wsbm.1240. Epub 2013 Sep 9. Wiley Interdiscip Rev Syst Biol Med. 2013. PMID: 24019266 Free PMC article. Review.
Cited by
- MicroRNAs shape the neuronal landscape.
McNeill E, Van Vactor D. McNeill E, et al. Neuron. 2012 Aug 9;75(3):363-79. doi: 10.1016/j.neuron.2012.07.005. Neuron. 2012. PMID: 22884321 Free PMC article. Review. - Human Neural Organoid Microphysiological Systems Show the Building Blocks Necessary for Basic Learning and Memory.
El Din DA, Moenkemoeller L, Loeffler A, Habibollahi F, Schenkman J, Mitra A, van der Molen T, Ding L, Laird J, Schenke M, Johnson EC, Kagan BJ, Hartung T, Smirnova L. El Din DA, et al. bioRxiv [Preprint]. 2024 Sep 19:2024.09.17.613333. doi: 10.1101/2024.09.17.613333. bioRxiv. 2024. PMID: 39345518 Free PMC article. Preprint. - Noise-induced collective dynamics in the small-world network of photosensitive neurons.
Li F, Li X, Ren L. Li F, et al. J Biol Phys. 2022 Sep;48(3):321-338. doi: 10.1007/s10867-022-09610-2. Epub 2022 Jul 25. J Biol Phys. 2022. PMID: 35879584 Free PMC article. - Dendritic spine morphology regulates calcium-dependent synaptic weight change.
Bell MK, Holst MV, Lee CT, Rangamani P. Bell MK, et al. J Gen Physiol. 2022 Aug 1;154(8):e202112980. doi: 10.1085/jgp.202112980. Epub 2022 Jul 12. J Gen Physiol. 2022. PMID: 35819365 Free PMC article. - Microbes, metabolites and (synaptic) malleability, oh my! The effect of the microbiome on synaptic plasticity.
Glinert A, Turjeman S, Elliott E, Koren O. Glinert A, et al. Biol Rev Camb Philos Soc. 2022 Apr;97(2):582-599. doi: 10.1111/brv.12812. Epub 2021 Nov 3. Biol Rev Camb Philos Soc. 2022. PMID: 34734461 Free PMC article. Review.
References
- Collins MO, et al. Proteomic analysis of in vivo phosphorylated synaptic proteins. J. Biol. Chem. 2005;280:5972–5982. - PubMed
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn. Mem. 2003;10:456–465. - PubMed
- Debanne D. Associative synaptic plasticity in hippocampus and visual cortex: cellular mechanisms and functional implications. Rev. Neurosci. 1996;7:29–46. - PubMed
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AA016022/AA/NIAAA NIH HHS/United States
- R01 AA018060/AA/NIAAA NIH HHS/United States
- R01 AA18060/AA/NIAAA NIH HHS/United States
- R01 AA16022/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous