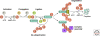Signaling to NF-kappaB: regulation by ubiquitination - PubMed (original) (raw)
Review
Signaling to NF-kappaB: regulation by ubiquitination
Ingrid E Wertz et al. Cold Spring Harb Perspect Biol. 2010 Mar.
Abstract
The NF-kappaB pathway is a ubiquitous stress response that activates the NF-kappaB family of transcription factors. Antigen receptors, receptors of the innate immune system, and certain intracellular stressors are potent activators of this pathway. The transcriptional program that is activated is both antiapoptotic and highly proinflammatory. Indeed, any compromise in engagement of the pathway results in immunodeficiency, whereas constitutive activation generates a sustained inflammatory response that may promote malignancy. As such, NF-kappaB activation is under tight regulation by a number of post-translational modifications, including phosphorylation and ubiquitination. This article attempts to synthesize our current knowledge regarding the regulation of NF-kappaB signaling by ubiquitination, specifically highlighting the biochemical basis for both positive and negative feedback loops that function in unison to generate coordinated signals that are essential for the viability of metazoan animals.
Figures
Figure 1.
Enzymes and reactions of the ubiquitin/proteasome system.
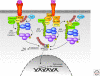
Figure 2.
TNFR1 and TLR4 signaling pathways. Lys-48-linked ubiquitin chains are shown in red, and Lys-63-linked ubiquitin chains are shown in green. See text for additional details.
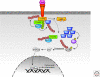
Figure 3.
CD40 signaling pathways. Lys-48-linked ubiquitin chains are shown in red, and Lys-63-linked ubiquitin chains are shown in green. See text for additional details.
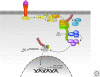
Figure 4.
TCR signaling pathways. Lys-48-linked ubiquitin chains are shown in red, and Lys-63-linked ubiquitin chains are shown in green. See text for additional details.
Figure 5.
NF-κB activation by intracellular receptors. Lys-48-linked ubiquitin chains are shown in red, Lys-63-linked ubiquitin chains are shown in green, SUMOylation is indicated by blue circles, and monoubiquitination is indicated by green circles. See text for additional details.
Similar articles
- Linear ubiquitination-mediated NF-κB regulation and its related disorders.
Tokunaga F. Tokunaga F. J Biochem. 2013 Oct;154(4):313-23. doi: 10.1093/jb/mvt079. Epub 2013 Aug 21. J Biochem. 2013. PMID: 23969028 Review. - Linear ubiquitination: a novel NF-κB regulatory mechanism for inflammatory and immune responses by the LUBAC ubiquitin ligase complex.
Tokunaga F, Iwai K. Tokunaga F, et al. Endocr J. 2012;59(8):641-52. doi: 10.1507/endocrj.ej12-0148. Epub 2012 May 19. Endocr J. 2012. PMID: 22673407 Review. - PDLIM7 Synergizes With PDLIM2 and p62/Sqstm1 to Inhibit Inflammatory Signaling by Promoting Degradation of the p65 Subunit of NF-κB.
Jodo A, Shibazaki A, Onuma A, Kaisho T, Tanaka T. Jodo A, et al. Front Immunol. 2020 Aug 4;11:1559. doi: 10.3389/fimmu.2020.01559. eCollection 2020. Front Immunol. 2020. PMID: 32849529 Free PMC article. - Protein Kinase-Mediated Decision Between the Life and Death.
Engin A. Engin A. Adv Exp Med Biol. 2021;1275:1-33. doi: 10.1007/978-3-030-49844-3_1. Adv Exp Med Biol. 2021. PMID: 33539010 - TNFR1-activated NF-κB signal transduction: regulation by the ubiquitin/proteasome system.
Wertz IE. Wertz IE. Curr Opin Chem Biol. 2014 Dec;23:71-7. doi: 10.1016/j.cbpa.2014.10.011. Curr Opin Chem Biol. 2014. PMID: 25461388 Review.
Cited by
- Ubc13: the Lys63 ubiquitin chain building machine.
Hodge CD, Spyracopoulos L, Glover JN. Hodge CD, et al. Oncotarget. 2016 Sep 27;7(39):64471-64504. doi: 10.18632/oncotarget.10948. Oncotarget. 2016. PMID: 27486774 Free PMC article. Review. - Deubiquitinases: Novel Therapeutic Targets in Immune Surveillance?
Lopez-Castejon G, Edelmann MJ. Lopez-Castejon G, et al. Mediators Inflamm. 2016;2016:3481371. doi: 10.1155/2016/3481371. Epub 2016 Aug 14. Mediators Inflamm. 2016. PMID: 27597804 Free PMC article. Review. - The unity of opposites: Strategic interplay between bacterial effectors to regulate cellular homeostasis.
Iyer S, Das C. Iyer S, et al. J Biol Chem. 2021 Dec;297(6):101340. doi: 10.1016/j.jbc.2021.101340. Epub 2021 Oct 23. J Biol Chem. 2021. PMID: 34695417 Free PMC article. Review. - A20 Establishes Negative Feedback With TRAF6/NF-κB and Attenuates Early Brain Injury After Experimental Subarachnoid Hemorrhage.
Deng HJ, Deji Q, Zhaba W, Liu JQ, Gao SQ, Han YL, Zhou ML, Wang CX. Deng HJ, et al. Front Immunol. 2021 Jul 26;12:623256. doi: 10.3389/fimmu.2021.623256. eCollection 2021. Front Immunol. 2021. PMID: 34381441 Free PMC article. - The immunoproteasomes regulate LPS-induced TRIF/TRAM signaling pathway in murine macrophages.
Reis J, Hassan F, Guan XQ, Shen J, Monaco JJ, Papasian CJ, Qureshi AA, Van Way CW 3rd, Vogel SN, Morrison DC, Qureshi N. Reis J, et al. Cell Biochem Biophys. 2011 Jun;60(1-2):119-26. doi: 10.1007/s12013-011-9183-7. Cell Biochem Biophys. 2011. PMID: 21455681 Free PMC article.
References
- Barton GM, Medzhitov R 2004. Toll signaling: RIPping off the TNF pathway. Nat Immunol 5:472–474 - PubMed
- Bertrand MJ, Doiron K, Labbe K, Korneluk RG, Barker PA, Saleh M 2009. Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity 30:789–801 - PubMed
- Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA 2008. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 30:689–700 - PubMed
- Bhoj VG, Chen ZJ 2009. Ubiquitylation in innate and adaptive immunity. Nature 458:430–437 - PubMed
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, et al.2004. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5:1052–1060 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials