GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism - PubMed (original) (raw)
GSK3 influences social preference and anxiety-related behaviors during social interaction in a mouse model of fragile X syndrome and autism
Marjelo A Mines et al. PLoS One. 2010.
Abstract
Background: Nearly 1% of children in the United States exhibit autism spectrum disorders, but causes and treatments remain to be identified. Mice with deletion of the fragile X mental retardation 1 (Fmr1) gene are used to model autism because loss of Fmr1 gene function causes Fragile X Syndrome (FXS) and many people with FXS exhibit autistic-like behaviors. Glycogen synthase kinase-3 (GSK3) is hyperactive in brains of Fmr1 knockout mice, and inhibition of GSK3 by lithium administration ameliorates some behavioral impairment in these mice. We extended our studies of this association by testing whether GSK3 contributes to socialization behaviors. This used two mouse models with disrupted regulation of GSK3, Fmr1 knockout mice and GSK3 knockin mice, in which inhibitory serines of the two isoforms of GSK3, GSK3alpha and GSK3beta, are mutated to alanines, leaving GSK3 fully active.
Methodology/principal findings: To assess sociability, test mice were introduced to a restrained stimulus mouse (S1) for 10 min, followed by introduction of a second restrained stimulus mouse (S2) for 10 min, which assesses social preference. Fmr1 knockout and GSK3 knockin mice displayed no deficit in sociability with the S1 mouse, but unlike wild-type mice neither demonstrated social preference for the novel S2 mouse. Fmr1 knockout mice displayed more anxiety-related behaviors during social interaction (grooming, rearing, and digging) than wild-type mice, which was ameliorated by inhibition of GSK3 with chronic lithium treatment.
Conclusions/significance: These results indicate that impaired inhibitory regulation of GSK3 in Fmr1 knockout mice may contribute to some socialization deficits and that lithium treatment can ameliorate certain socialization impairments. As discussed in the present work, these results suggest a role for GSK3 in social behaviors and implicate inhibition of GSK3 as a potential therapeutic.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
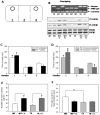
Figure 1. Influences of lithium treatment on the sociability of Fmr1 knockout and wild-type mice.
A. Diagram of the social interaction apparatus. The ovals represent the wire enclosures used to secure the stimulus mice, S1 in Chamber 1 and S2 in Chamber 3. The breaks in the center walls represent the circular openings allowing the mice to move between chambers. B. Representative image of genotyping results from wild-type and Fmr1 knockout mice (top). Chronic lithium treatment increased inhibitory serine-phosphorylation of GSK3 (PS-GSK3) on serine-21 of GSK3α and on serine-9 of GSK3β in the hippocampus of wild-type and Fmr1 knockout mice (bottom) as reported previously , . C. Mean total time spent by wild-type mice in each chamber during the sociability period. Wild-type (WT) (n = 9) mice spent significantly more time in Chamber 1, the chamber with S1, compared to empty Chamber 2 or Chamber 3 with the wire enclosure only. Chronic lithium treatment of WT mice (n = 5) had no significant effect on sociability. * p<0.05 comparing time spent in Chamber 1 with time in Chambers 2 and 3. D. Mean total time spent by Fmr1 knockout mice in each chamber during the sociability period. Fmr1 knockout (FX) mice (n = 9) spent significantly more time in Chamber 1, the chamber with S1, compared to empty Chamber 2 or Chamber 3 with the wire enclosure only. FX mice (n = 6) chronically treated with lithium displayed a significant increase in time spent in Chamber 1, with S1, as compared to time in Chambers 2 or 3. Treated mice also displayed a significant increase in time spent in Chamber 1 and a significant decrease in time spent in Chamber 3, as compared to untreated controls. * p<0.05 comparing time spent in Chamber 1 with Chambers 2 and 3; # p<0.05 compared to untreated FX mice. E. The total number of nose contacts by the test mouse with S1 was equivalent for untreated WT and FX mice. Chronic lithium treatment significantly increased the number of nose contacts for both WT and FX mice. * p<0.05 compared to untreated group mates. F. The duration of nose contacts with S1 was lower in FX than WT mice. Lithium treatment had no effect on social interaction (defined as the average duration of nose contacts). * p<0.05 compared to untreated WT mice.
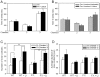
Figure 2. Fmr1 knockout mice exhibit impairments in social preference.
A. Mean total time spent by wild-type mice in each chamber during the social preference period. Wild-type (WT) mice (n = 9) spent significantly more time in Chamber 3 with the novel S2 mouse compared to empty Chamber 2, or to Chamber 1 with the familiar S1 mouse. Chronic lithium treatment did not significantly alter time spent in each chamber (n = 5). * p<0.05 comparing time spent in Chamber 3 with Chambers 1 and 2. B. Mean total time spent by Fmr1 knockout mice in each chamber during the social preference period. There was not a significant difference in the amount of time Fmr1 knockout (FX) mice (n = 9) spent in Chamber 3 with the novel mouse S2 and in Chamber 1 with the familiar mouse S1. Chronic lithium treatment did not significantly alter time spent in each chamber (n = 6). C. WT mice, but not FX mice, displayed a significant preference for nose contacts with S2, compared to S1. Chronic lithium treatment significantly increased the number of nose contacts with S2 by both WT and FX mice. * p<0.05 nose contacts with S2 compared with S1; ** p<0.05 compared to nose contacts with S2 by untreated group mates. D. The duration of nose contacts with S1 and S2 was not significantly different between WT and FX mice. Chronic lithium treatment had no significant effect on nose contact duration.
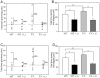
Figure 3. Fmr1 knockout mice exhibit increased social anxiety-related behaviors during social interaction.
A. During the sociability period, there was not a significant difference in grooming times of Fmr1 knockout (FX) mice and wild-type (WT) mice. Chronic lithium treatment had no significant effect on WT grooming times but slightly decreased the percentage of FX mice with grooming times above the median time. Dashed lines represent median grooming times, 12 sec and 12.5 sec for WT and FX mice, respectively. B. FX mice spent significantly more time rearing and digging than WT mice during the sociability period. Chronic lithium treatment significantly decreased duration of rearing and digging behaviors in both groups of mice. *p<0.05 compared to untreated group mates; ** p<0.05 comparing untreated WT and FX mice. C. During the social preference period, there was not a significant difference in grooming times of Fmr1 knockout (FX) mice and wild-type (WT) mice. Chronic lithium treatment had no significant effect on grooming times, but slightly decreased the percentage of WT and FX mice with grooming times above the median time. Dashed lines represent median grooming times, 8.5 sec and 11 sec for WT and FX mice, respectively. D. FX mice spent significantly more time rearing and digging than WT mice during the social preference period. Chronic lithium treatment significantly decreased duration of rearing and digging behaviors in both groups of mice. * p<0.05 compared to untreated group mates; ** p<0.05 comparing untreated WT and FX mice.
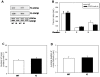
Figure 4. Sociability is not altered in GSK3 knockin mice.
A. GSK3 knockin mice lack serine-phosphorylation of GSK3 (PS-GSK3) on serine-21 of GSK3α and on serine-9 of GSK3β, which is evident in wild-type mice, as reported previously , . Total GSK3 levels are equivalent in GSK3 knockin mice and wild-type mice. B. Mean total time spent in each chamber during the sociability period. Wild-type (WT) (n = 7) mice and GSK3 knockin (KI) (n = 10) mice spent significantly more time in Chamber 1, the chamber with S1, compared to empty Chamber 2 or Chamber 3 with the wire enclosure only. * p<0.05 comparing time spent in Chamber 1 with Chambers 2 and 3. C. Social approach, the total number of nose contacts by the test mouse with S1, was equivalent for untreated WT and KI mice. D. There was no difference in social interaction between WT and KI mice.
Figure 5. GSK3 knockin mice exhibit impairments in social preference.
A. Mean total time spent in each chamber during the social preference period. Wild-type (WT) mice (n = 11), but not GSK3 knockin (KI) mice (n = 10), spent significantly more time in Chamber 3 with the novel S2 mouse compared to empty Chamber 2, or to Chamber 1 with the familiar (S1) mouse. * p<0.05 comparing time spent in Chamber 3 with Chambers 1 and 2. B. GSK3 KI mice display deficits in social approach preference, as indicated by no significant difference in the number of nose contacts with S1 or S2. C. The duration of nose contacts with S2 was significantly greater than with S1 for WT, but not GSK3 KI, mice. * p<0.05 comparing WT duration of nose contacts with S1 and S2.
Figure 6. Anxiety-related behaviors during social interaction in GSK3 knockin mice.
A. During the sociability period, there was not a significant difference in grooming times of GSK3 knockin (KI) mice and wild-type (WT) mice, but the percentage of wild-type (WT) mice with grooming times above the median was higher than GSK3 knockin (KI) mice. Dashed lines represent median grooming times, 12.5 sec. B. GSK3 KI mice spent significantly less time rearing and digging than WT mice during the sociability period. * p<0.05 compared to untreated WT mice. C. During the social preference period, there was not a significant difference in grooming times of GSK3 knockin (KI) mice and wild-type (WT) mice, but the percentage of KI mice with grooming times above the median was slightly higher than WT controls. Dashed lines represent median grooming times, 12.5 sec. D. WT and GSK3 KI mice spent similar times rearing and digging during the social preference period.
Similar articles
- Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome.
Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Yuskaitis CJ, et al. Biochem Pharmacol. 2010 Feb 15;79(4):632-46. doi: 10.1016/j.bcp.2009.09.023. Biochem Pharmacol. 2010. PMID: 19799873 Free PMC article. - Evidence of reactive astrocytes but not peripheral immune system activation in a mouse model of Fragile X syndrome.
Yuskaitis CJ, Beurel E, Jope RS. Yuskaitis CJ, et al. Biochim Biophys Acta. 2010 Nov;1802(11):1006-12. doi: 10.1016/j.bbadis.2010.06.015. Epub 2010 Jul 1. Biochim Biophys Acta. 2010. PMID: 20600866 Free PMC article. - Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome.
Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Spencer CM, et al. Genes Brain Behav. 2005 Oct;4(7):420-30. doi: 10.1111/j.1601-183X.2005.00123.x. Genes Brain Behav. 2005. PMID: 16176388 - Social behavior phenotypes in fragile X syndrome, autism, and the Fmr1 knockout mouse: theoretical comment on McNaughton et al. (2008).
Brodkin ES. Brodkin ES. Behav Neurosci. 2008 Apr;122(2):483-9. doi: 10.1037/0735-7044.122.2.483. Behav Neurosci. 2008. PMID: 18410188 Review. - Learning and behavioral deficits associated with the absence of the fragile X mental retardation protein: what a fly and mouse model can teach us.
Santos AR, Kanellopoulos AK, Bagni C. Santos AR, et al. Learn Mem. 2014 Sep 16;21(10):543-55. doi: 10.1101/lm.035956.114. Print 2014 Oct. Learn Mem. 2014. PMID: 25227249 Free PMC article. Review.
Cited by
- Downregulating the canonical Wnt/β-catenin signaling pathway attenuates the susceptibility to autism-like phenotypes by decreasing oxidative stress.
Zhang Y, Sun Y, Wang F, Wang Z, Peng Y, Li R. Zhang Y, et al. Neurochem Res. 2012 Jul;37(7):1409-19. doi: 10.1007/s11064-012-0724-2. Epub 2012 Feb 29. Neurochem Res. 2012. PMID: 22374471 - Brief report: altered social behavior in isolation-reared Fmr1 knockout mice.
Heitzer AM, Roth AK, Nawrocki L, Wrenn CC, Valdovinos MG. Heitzer AM, et al. J Autism Dev Disord. 2013 Jun;43(6):1452-8. doi: 10.1007/s10803-012-1670-1. J Autism Dev Disord. 2013. PMID: 23015112 - The molecular basis of cognitive deficits in pervasive developmental disorders.
Bhattacharya A, Klann E. Bhattacharya A, et al. Learn Mem. 2012 Aug 16;19(9):434-43. doi: 10.1101/lm.025007.111. Learn Mem. 2012. PMID: 22904374 Free PMC article. Review. - Investigation of gene effects and epistatic interactions between Akt1 and neuregulin 1 in the regulation of behavioral phenotypes and social functions in genetic mouse models of schizophrenia.
Huang CH, Pei JC, Luo DZ, Chen C, Chen YW, Lai WS. Huang CH, et al. Front Behav Neurosci. 2015 Jan 29;8:455. doi: 10.3389/fnbeh.2014.00455. eCollection 2014. Front Behav Neurosci. 2015. PMID: 25688191 Free PMC article. - Programming social behavior by the maternal fragile X protein.
Zupan B, Sharma A, Frazier A, Klein S, Toth M. Zupan B, et al. Genes Brain Behav. 2016 Jul;15(6):578-87. doi: 10.1111/gbb.12298. Epub 2016 Jun 10. Genes Brain Behav. 2016. PMID: 27198123 Free PMC article.
References
- Bitterman A, Daley TC, Misra S, Carlson E, Markowitz J. A national sample of preschoolers with autism spectrum disorders: special education services and parent satisfaction. J Autism Dev Disord. 2008;38:1509–1517. - PubMed
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. - PubMed
- Kau AS, Tierney E, Bukelis I, Stump MH, Kates WR, et al. Social behavior profile in young males with fragile X syndrome: characteristics and specificity. Am J Med Genet A. 2004;126:9–17. - PubMed
- Hagerman RJ, Ono MY, Hagerman PJ. Recent advances in fragile X: a model for autism and neurodegeneration. Curr Opin Psychiatry. 2005;18:490–496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials