Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis - PubMed (original) (raw)
. 2010 Mar 26;32(3):414-25.
doi: 10.1016/j.immuni.2010.03.004. Epub 2010 Mar 18.
Cengiz Zubeyir Altuntas, Muhammet Fatih Gulen, Caini Liu, Natalia Giltiay, Hongwei Qin, Liping Liu, Wen Qian, Richard M Ransohoff, Cornelia Bergmann, Stephen Stohlman, Vincent K Tuohy, Xiaoxia Li
Affiliations
- PMID: 20303295
- PMCID: PMC3073618
- DOI: 10.1016/j.immuni.2010.03.004
Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis
Zizhen Kang et al. Immunity. 2010.
Abstract
Interleukin-17 (IL-17) secreted by T helper 17 (Th17) cells is essential in the development of experimental autoimmune encephalomyelitis (EAE). However, it remains unclear how IL-17-mediated signaling in different cellular compartments participates in the central nervous system (CNS) inflammatory process. We examined CNS inflammation in mice with specific deletion of Act1, a critical component required for IL-17 signaling, in endothelial cells, macrophages and microglia, and neuroectoderm (neurons, astrocytes, and oligodendrocytes). In Act1-deficient mice, Th17 cells showed normal infiltration into the CNS but failed to recruit lymphocytes, neutrophils, and macrophages. Act1 deficiency in endothelial cells or in macrophages and microglia did not substantially impact the development of EAE. However, targeted Act1 deficiency in neuroectoderm-derived CNS-resident cells resulted in markedly reduced severity in EAE. Specifically, Act1-deficient astrocytes showed impaired IL-17-mediated inflammatory gene induction. Thus, astroctyes are critical in IL-17-Act1-mediated leukocyte recruitment during autoimmune-induced inflammation of the CNS.
Figures
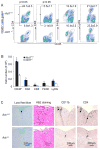
Figure 1. Act1 deficiency impairs inflammatory cell infiltration in the CNS
(A–B) Immune cell infiltration in the brains of MOG35-55 immunized wild-type and _Act1_−/− mice (n=7, 7 days after disease onset) was analyzed by flow cytometry. Error bars, SEM *, p<0.05. (C) Luxol fast blue, hematoxylin and eosin, and anti-CD11b and CD4 staining of spinal cores of wild-type and Act1-deficient mice at peak of disease. Data are representative of three independent experiments. See also Figure S1.
Figure 2. Act1 is required for the effector stage of Th17-, but not Th1-induced EAE
(A) Draining lymph node cells from wild-type mice and _Act1_−/− mice 10 days after immunization with MOG35-55 were re-stimulated with MOG35-55 in vitro for 4 days, followed by ELISA of IL-17, IFN-γ. Error bars, SEM; n = 5 mice per group. *, p<0.05. (B–D) Primed MOG35-55 specific T cells (10 days) from (B) wild-type or (C) _Act1_−/− mice were re-stimulated with MOG35-55 in vitro in the presence of recombinant IL-23 for 5 days, and then transferred to naïve wild-type or _Act1_−/− mice. Graph represents the average clinical score after T-cell transfer. (D) Primed MOG35-55 specific wild-type T cells (10 days) were re-stimulated with MOG35-55 in vitro in the presence of recombinant IL-12 and anti-IL-23p19 for 5 days, and then transferred to naïve wild-type and _Act1_−/− mice. Graph represents the average clinical score after T-cell transfer. (E) H&E staining of the spinal cords of wild-type and _Act1_−/− mice transferred with Th1 and Th17 cells and sacrificed 7 days after the onset of disease. (F) Immune cell infiltration in the brains of wild-type and _Act1_−/− mice transferred with wild-type Th1 cells (n=5, 7 days after disease onset) was analyzed by flow cytometry. Error bars, SEM. Data are representative of three independent experiments. See also Figure S2.
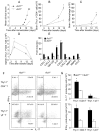
Figure 3. Th17 cells can infiltrate CNS but fail to initiate effective inflammatory cascade in _Act1_−/− mice
(A) Mean clinical score of wild-type and _Act1_−/− mice adoptively transferred with MOG-specific Thy1.1+ Th17 cells (n=4/group). Lymph node cells harvested from Thy1.1 C57BL/6 mice 10 days after immunization with MOG35-55 were polarized to Th17 cells as described in Figure 2, and transferred to wild-type and _Act1_−/− mice. Graph represents the average clinical score after T-cell transfer. (B–D) Immune cell infiltration in the (B,C) brain and(D) spleen of wild-type and _Act1_−/− mice transferred with MOG-specific Thy1.1+ Th17 cells (n=5/group, 3, 5, 12, and 15 days after T cell transfer) was analyzed by flow cytometry. Error bars, SEM; *, p<0.05. (E) Real-time PCR analysis of relative expression of inflammatory genes as indicated in spinal cords of wild-type and _Act1_−/− mice transferred with MOG-specific Thy1.1+ Th17 cells (n=3, 15 days after T cell transfer) compared to the control samples from naïve mice. (F–G) Intracellular staining for the infiltrated IL-17-secreting or IFN-γ-secreting Thy1.1+CD4+T cells and Thy1.1+γδT cells in the brain of wild-type and _Act1_−/− mice transferred with MOG-specific Thy1.1+ Th17 cells (n=10/group, 15 days after T cell transfer). The frequency of IL-17+ or IFN-γ+ cells in Thy1.1+CD4+ cell population (upper panel) and Thy1.1+ γδT cells (lower panel) were shown in (F) and the corresponding absolute cell numbers were shown in G. See also Figure S2.
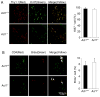
Figure 4. Act1 deficiency in the recipient mice does not impact on the proliferation and survival of the infiltrated Th17 cells
(A) Immunofluorescent staining for Ki67-positive Thy1.1 cells in the spinal cords of wild-type and Act1-deficient mice transferred with MOG-specific Thy1.1+ Th17 cells (n=4), 12 days after T cell transfer). Frozen sections of spinal cords collected from the recipient mice were stained with Thy1.1 (red) and Ki67 (green) antibodies. The presented frequency of Ki67+ cells in total Thy1.1+ cells in the spinal cord was an average of four spinal cord regions each group. Error bars, SEM. (B) Brdu incorporation of infiltrated T cells in the spinal cords of wild-type and _Act1_−/− mice transferred with MOG-specific Thy1.1+ Th17 cells (n=4), 12 days after T cell transfer). On day 12 after adoptive transfer of MOG-specific Thy1.1+ Th17 cells, wild-type and _Act1_−/− recipient were administrated with Brdu (i.p., 1mg/mouse). Spinal cords were collected from the recipient mice 12 h after Brdu administration and stained with Brdu antibody (red) and CD4 antibody (green). The presented percentage of Brdu-positive cells in total CD4+ cells was an average of four spinal cord regions each group. Error bars, SEM.
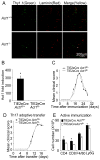
Figure 5. Endothelial-derived Act1 is dispensable for EAE development
(A) Immunofluorescent staining of CD4+ T cells relative to the vasculature in the spinal cord. Spinal cords of wild-type and _Act1_−/− mice transferred with MOG-specific Thy1.1+ Th17 cells (n=3, 12 days after T cell transfer) were stained with CD4 (green) and pan-laminin (red) antibodies. The presented data is a representative of three independent experiments. (B) Real-time PCR for the Act1 expression in PECAM-1+ heart endothelial cells from endothelial-specific Act1-deficient (TIE2eCre_Act1_fl/−) and control mice (TIE2eCre_Act1_fl/+). (C) Mean clinical score of EAE in TIE2eCreAct1fl/− and TIE2eCreAct1fl/+ induced by active immunization with MOG35-55 (n=5/group). (D) Mean clinical score of EAE in TIE2eCreAct1fl/− and TIE2eCreAct1fl/+ mice induced by MOG-specific wild-type Th17 cells. (E) Flow cytometry analysis of immune cell infiltration in the brains of MOG35-55 immunized TIE2eCreAct1fl/− and TIE2eCreAct1fl/+ mice (n=5, 14 days after disease onset). Error bars, SEM; *, p<0.05.
Figure 6. Macrophage- and Microglial-derived Act1 are dispensable for EAE development
(A) Real-time PCR for the Act1expression in bone marrow-derived macrophages and microglia from the macrophage/microglia-specific Act1-deficient (CD11bCre_Act1_fl/−) and control mice (CD11bCre_Act1_fl/+). (B) Immunoblot analysis for the Act1 expression in bone marrow-derived macrophages from the macrophage and microglia-specific Act1-deficient (CD11bCre_Act1_fl/−) and control mice (CD11bCre_Act1_fl/+). (C) Mean clinical score of EAE in CD11bCre Act1fl/− and CD11bCre Act1fl/+ mice induced by active immunization with MOG35-55 (n=5/group). (D) Mean clinical score of EAE in the CD11bCre_Act1_fl/− and CD11bCre_Act1_fl/+ mice induced by MOG-specific Th17 cells. (E) Flow cytometry analysis of immune cell infiltration in the brains of MOG35-55 immunized CD11bCre_Act1_fl/− and CD11bCre_Act1_fl/+ mice (n=5, 7 days after disease onset). Error bars, SEM; *, p<0.05.
Figure 7. Amelioration of EAE by targeting Act1 in CNS resident cells derived from neuroectodermal cells
(A) Immunoblot analysis for the Act1 expression in the brains and astrocytes from CNS-restricted Act1-deficient (NesCre_Act1_fl/−) and control mice (NesCre_Act1_fl/+). (B) Mean clinical score of EAE in NesCre_Act1_fl/− and NesCre_Act1_fl/+ mice induced by active immunization with MOG35-55. (C) Luxol fast blue, anti-CD11b and anti-CD4 staining of spinal cores of NesCre_Act1_fl/− and NesCre_Act1_fl/+ mice after immunization with MOG35-55 (n=5, 7 days after disease onset). Data are representative of three independent experiments. (D) Mean clinical score of Th1-induced EAE (upper panel) and flow cytometry analysis of immune cell infiltration in the brains (lower panel) of NesCre_Act1_fl/− and NesCre_Act1_fl/+ mice (n=5, 15 days after T cell transfer). (E) Mean clinical score of Th17-induced EAE (upper panel) and flow cytometry analysis of immune cell infiltration in the brains (lower panel) of NesCre_Act1_fl/− and NesCre_Act1_fl/+ mice (n=5, 15 days after T cell transfer). (F) Real-time PCR analysis of relative expression of inflammatory genes as indicated in spinal cords of NesCre_Act1_fl/− and NesCre_Act1_fl/+ mice transferred with MOG-specific Th17 cells (n=3, 15 days after T cell transfer) compared to the control samples from naïve mice. (G) Primary astrocytes from NesCre_Act1_fl/− and NesCre_Act1_fl/+ mice were treated with 50 ng/ml IL-17 for the indicated times and analyzed by immunoblot with indicated antibodies. (H) Differential regulation of chemokines by IL-17 and IFN-γ in astrocytes Astrocytes from NesCre_Act1_fl/− and NesCre_Act1_fl/+ mice were treated with 50ng/ml IL-17, 10ng/ml TNF-α, 10ng/ml IFN-γ, medium alone, IL-17+ TNF-α and TNF-α+IFN-γ for 16hr, followed by real-time PCR for the expression of inflammatory genes as indicated. The data are presented as mean±SEM from three independent experiments (*p<0.05). See also Figure S3–S4.
Comment in
- Act1, scene brain: astrocytes play a lead role.
Rodgers JM, Zhou L, Miller SD. Rodgers JM, et al. Immunity. 2010 Mar 26;32(3):302-4. doi: 10.1016/j.immuni.2010.03.009. Immunity. 2010. PMID: 20346771 Free PMC article.
Similar articles
- Act1, scene brain: astrocytes play a lead role.
Rodgers JM, Zhou L, Miller SD. Rodgers JM, et al. Immunity. 2010 Mar 26;32(3):302-4. doi: 10.1016/j.immuni.2010.03.009. Immunity. 2010. PMID: 20346771 Free PMC article. - CNS-specific therapy for ongoing EAE by silencing IL-17 pathway in astrocytes.
Yan Y, Ding X, Li K, Ciric B, Wu S, Xu H, Gran B, Rostami A, Zhang GX. Yan Y, et al. Mol Ther. 2012 Jul;20(7):1338-48. doi: 10.1038/mt.2012.12. Epub 2012 Mar 20. Mol Ther. 2012. PMID: 22434134 Free PMC article. - RKIP mediates autoimmune inflammation by positively regulating IL-17R signaling.
Lin W, Wang N, Zhou K, Su F, Jiang Y, Shou J, Liu H, Ma C, Qian Y, Wang K, Wang X. Lin W, et al. EMBO Rep. 2018 Jun;19(6):e44951. doi: 10.15252/embr.201744951. Epub 2018 Apr 19. EMBO Rep. 2018. PMID: 29674348 Free PMC article. - IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease.
Zepp J, Wu L, Li X. Zepp J, et al. Trends Immunol. 2011 May;32(5):232-9. doi: 10.1016/j.it.2011.02.007. Epub 2011 Apr 12. Trends Immunol. 2011. PMID: 21493143 Free PMC article. Review. - Translational mini-review series on Th17 cells: are T helper 17 cells really pathogenic in autoimmunity?
Koenders MI, van den Berg WB. Koenders MI, et al. Clin Exp Immunol. 2010 Feb;159(2):131-6. doi: 10.1111/j.1365-2249.2009.04039.x. Epub 2009 Nov 11. Clin Exp Immunol. 2010. PMID: 19912250 Free PMC article. Review.
Cited by
- From Physiology to Pathology of Astrocytes: Highlighting Their Potential as Therapeutic Targets for CNS Injury.
Yuan Y, Liu H, Dai Z, He C, Qin S, Su Z. Yuan Y, et al. Neurosci Bull. 2024 Jul 30. doi: 10.1007/s12264-024-01258-3. Online ahead of print. Neurosci Bull. 2024. PMID: 39080102 Review. - Transcriptome Analysis of the Striatum of Electroacupuncture-treated Naïve and Ischemic Stroke Mice.
Lee HJ, Shin HK, Kim JH, Choi BT. Lee HJ, et al. J Pharmacopuncture. 2024 Jun 30;27(2):162-171. doi: 10.3831/KPI.2024.27.2.162. J Pharmacopuncture. 2024. PMID: 38948311 Free PMC article. - The Role of Selected Interleukins in the Development and Progression of Multiple Sclerosis-A Systematic Review.
Grunwald C, Krętowska-Grunwald A, Adamska-Patruno E, Kochanowicz J, Kułakowska A, Chorąży M. Grunwald C, et al. Int J Mol Sci. 2024 Feb 23;25(5):2589. doi: 10.3390/ijms25052589. Int J Mol Sci. 2024. PMID: 38473835 Free PMC article. Review. - Mechanisms of immune response and cell death in ischemic stroke and their regulation by natural compounds.
Gong Z, Guo J, Liu B, Guo Y, Cheng C, Jiang Y, Liang N, Hu M, Song T, Yang L, Li H, Zhang H, Zong X, Che Q, Shi N. Gong Z, et al. Front Immunol. 2024 Jan 11;14:1287857. doi: 10.3389/fimmu.2023.1287857. eCollection 2023. Front Immunol. 2024. PMID: 38274789 Free PMC article. Review. - The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders.
Knezevic E, Nenic K, Milanovic V, Knezevic NN. Knezevic E, et al. Cells. 2023 Nov 29;12(23):2726. doi: 10.3390/cells12232726. Cells. 2023. PMID: 38067154 Free PMC article. Review.
References
- Agrawal A, Dillon S, Denning TL, Pulendran B. ERK1−/− mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2006;176:5788–5796. - PubMed
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. - PubMed
- Awane M, Andres PG, Li DJ, Reinecker HC. NF-kappa B-inducing kinase is a common mediator of IL-17-, TNF-alpha-, and IL-1 beta-induced chemokine promoter activation in intestinal epithelial cells. J Immunol. 1999;162:5337–5344. - PubMed
- Becher B, Bechmann I, Greter M. Antigen presentation in autoimmunity and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med. 2006;84:532–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL098935/HL/NHLBI NIH HHS/United States
- R01 CA140350/CA/NCI NIH HHS/United States
- R01 AI065470/AI/NIAID NIH HHS/United States
- R01 AI065470-05/AI/NIAID NIH HHS/United States
- P01 HL103453/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases