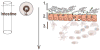Homeostasis and inflammation in the intestine - PubMed (original) (raw)
Review
Homeostasis and inflammation in the intestine
Wendy S Garrett et al. Cell. 2010.
Abstract
The gut is home to our largest collection of microbes. The ability of the immune system to coevolve with the microbiota during postnatal life allows the host and microbiota to coexist in a mutually beneficial relationship. Failure to achieve or maintain equilibrium between a host and its microbiota has negative consequences for both intestinal and systemic health. In this Review, we consider the many cellular and molecular methods by which inflammatory responses are regulated to maintain intestinal homeostasis and the disease states that can ensue when this balance is lost.
2010 Elsevier Inc. All rights reserved.
Figures
Figure 1. The gut landscape: Maintaining intestinal homeostasis
The mucus layer, sitting atop the intestinal epithelium, is a key component of the mucosal barrier and also is both a source of nutrients and a microhabitat for bacterial members of the microbiota. The epithelial crypt-villus axis differs between the small and large intestine. The populating enterocyte populations vary as well. M cells and Paneth cells are restricted to the small intestine. Intestinal immune cells that mediate tolerance-inducing responses and participate in host defense, localize to inductive sites. These sites include Peyers patches (small intestine), lymphoid follicles, and colonic patches (large intestine) and effector sites such as the epithelium and underlying lamina propria.
Figure 2. Regulators of host-microbial interactions in the gut
The commensal microbiota, intestinal epithelial cells, and intestinal immune cells engage in a complex crosstalk. Epithelial cells, M cells, and dendritic cells (DCs) can directly sense and sample the intestinal contents and communicate information about the microbiota to other subsets of immune cells. The Toll-like receptors are one class of microbe-sensing molecules expressed by epithelial cells, M cells and DCs. Cytokines, chemokines, and host and microbial metabolites are key molecular mediators of intestinal homeostasis that influence responses of both host and microbe.
Similar articles
- Microbes, intestinal inflammation and probiotics.
Khan MW, Kale AA, Bere P, Vajjala S, Gounaris E, Pakanati KC. Khan MW, et al. Expert Rev Gastroenterol Hepatol. 2012 Feb;6(1):81-94. doi: 10.1586/egh.11.94. Expert Rev Gastroenterol Hepatol. 2012. PMID: 22149584 Review. - Host-microbiota interactions in inflammatory bowel disease.
Caruso R, Lo BC, Núñez G. Caruso R, et al. Nat Rev Immunol. 2020 Jul;20(7):411-426. doi: 10.1038/s41577-019-0268-7. Epub 2020 Jan 31. Nat Rev Immunol. 2020. PMID: 32005980 Review. - Regulation of intestinal homeostasis and immunity with probiotic lactobacilli.
van Baarlen P, Wells JM, Kleerebezem M. van Baarlen P, et al. Trends Immunol. 2013 May;34(5):208-15. doi: 10.1016/j.it.2013.01.005. Epub 2013 Feb 26. Trends Immunol. 2013. PMID: 23485516 Review. - Interactions between intestinal microbiota and innate immune system in pediatric inflammatory bowel disease.
Cucchiara S, Stronati L, Aloi M. Cucchiara S, et al. J Clin Gastroenterol. 2012 Oct;46 Suppl:S64-6. doi: 10.1097/MCG.0b013e31826a857f. J Clin Gastroenterol. 2012. PMID: 22955361 Retracted. - Control of intestinal homeostasis through crosstalk between natural killer T cells and the intestinal microbiota.
Dowds CM, Blumberg RS, Zeissig S. Dowds CM, et al. Clin Immunol. 2015 Aug;159(2):128-33. doi: 10.1016/j.clim.2015.05.008. Epub 2015 May 16. Clin Immunol. 2015. PMID: 25988859 Free PMC article. Review.
Cited by
- The Wnt5a-Ror2 axis promotes the signaling circuit between interleukin-12 and interferon-γ in colitis.
Sato A, Kayama H, Shojima K, Matsumoto S, Koyama H, Minami Y, Nojima S, Morii E, Honda H, Takeda K, Kikuchi A. Sato A, et al. Sci Rep. 2015 Jun 1;5:10536. doi: 10.1038/srep10536. Sci Rep. 2015. PMID: 26030277 Free PMC article. - Protective Effect and Mechanism of Aspirin Eugenol Ester on Lipopolysaccharide-Induced Intestinal Barrier Injury.
Tao Q, Liu XW, Zhang ZD, Ma N, Lu XR, Ge WB, Li JY, Yang YJ. Tao Q, et al. Int J Mol Sci. 2023 Dec 13;24(24):17434. doi: 10.3390/ijms242417434. Int J Mol Sci. 2023. PMID: 38139262 Free PMC article. - Focused examination of the intestinal lamina propria yields greater molecular insight into mechanisms underlying SIV induced immune dysfunction.
Mohan M, Kaushal D, Aye PP, Alvarez X, Veazey RS, Lackner AA. Mohan M, et al. PLoS One. 2012;7(4):e34561. doi: 10.1371/journal.pone.0034561. Epub 2012 Apr 12. PLoS One. 2012. PMID: 22511950 Free PMC article. - Regulatory immune cells in regulation of intestinal inflammatory response to microbiota.
Sun M, He C, Cong Y, Liu Z. Sun M, et al. Mucosal Immunol. 2015 Sep;8(5):969-978. doi: 10.1038/mi.2015.49. Epub 2015 Jun 17. Mucosal Immunol. 2015. PMID: 26080708 Free PMC article. Review. - Germs gone wild.
Hoshi N, Medzhitov R. Hoshi N, et al. Nat Med. 2012 May 4;18(5):654-6. doi: 10.1038/nm.2767. Nat Med. 2012. PMID: 22561817 Free PMC article.
References
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8:411–420. - PubMed
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. - PubMed
Publication types
MeSH terms
Grants and funding
- AI32412/AI/NIAID NIH HHS/United States
- R01 CA112663-10/CA/NCI NIH HHS/United States
- R01 CA112663/CA/NCI NIH HHS/United States
- CA112663/CA/NCI NIH HHS/United States
- R01 AI032412/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical