Immunity, inflammation, and cancer - PubMed (original) (raw)
Review
Immunity, inflammation, and cancer
Sergei I Grivennikov et al. Cell. 2010.
Abstract
Inflammatory responses play decisive roles at different stages of tumor development, including initiation, promotion, malignant conversion, invasion, and metastasis. Inflammation also affects immune surveillance and responses to therapy. Immune cells that infiltrate tumors engage in an extensive and dynamic crosstalk with cancer cells, and some of the molecular events that mediate this dialog have been revealed. This review outlines the principal mechanisms that govern the effects of inflammation and immunity on tumor development and discusses attractive new targets for cancer therapy and prevention.
2010 Elsevier Inc. All rights reserved.
Figures
Figure 1
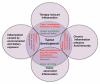
Types of inflammation in tumorigenesis and cancer. Chronic inflammation associated with infections or autoimmune disease precedes tumor development and can contribute to it through induction of oncogenic mutations, genomic instability, early tumor promotion, and enhanced angiogenesis. Prolonged exposure to environmental irritants or obesity can also result in low-grade chronic inflammation that precedes tumor development and contributes to it through the mechanisms mentioned above. Tumor-associated inflammation goes hand in hand with tumor development. This inflammatory response can enhance neo-angiogenesis, promote tumor progression and metastatic spread, cause local immunosuppression, and further augment genomic instability. Cancer therapy can also trigger an inflammatory response by causing trauma, necrosis, and tissue injury that stimulate tumor re-emergence and resistance to therapy. However, in some cases, therapy-induced inflammation can enhance antigen presentation, leading to immune-mediated tumor eradication. Tumor promoting mechanisms are in red and anti-tumorigenic mechanisms are in green.
Figure 2
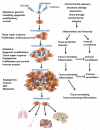
The multifaceted role of inflammation in cancer Inflammation acts at all stages of tumorigenesis. It may contribute to tumor initiation through mutations, genomic instability, and epigenetic modifications. Inflammation activates tissue repair responses, induces proliferation of premalignant cells, and enhances their survival. Inflammation also stimulates angiogenesis, causes localized immunosuppression, and promotes the formation of a hospitable microenvironment in which pre-malignant cells can survive, expand, and accumulate additional mutations and epigenetic changes. Eventually, inflammation also promotes metastatic spread. Mutated cells are marked with “X”. Yellow - stromal cells, Brown - malignant cells, Red - blood vessels, Blue - immune and inflammatory cells. Epithelial-mesenchymal transition, EMT; reactive oxygen species, ROS; reactive nitrogen intermediates (RNI)
Figure 3
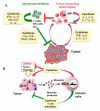
Role of inflammation in tumor initiation and promotion A) Tumor initiation. Reactive oxygen species (ROS) and reactive nitrogen intermediates (RNI) produced by inflammatory cells may cause mutations in neighboring epithelial cells. Also, cytokines produced by inflammatory cells can elevate intracellular ROS and RNI in pre-malignant cells. In addition, inflammation can result in epigenetic changes that favor tumor initiation. Tumor-associated inflammation contributes to further ROS, RNI and cytokine production. B) Tumor promotion. Cytokines produced by tumor infiltrating immune cells activate key transcription factors, such as NF-κB or STAT3, in pre-malignant cells to control numerous pro-tumorigenic processes, including survival, proliferation, growth, angiogenesis, and invasion. As parts of positive feed-forward loops, NF-κB and STAT3 induce production of chemokines that attract additional immune/inflammatory cells to sustain tumor-associated inflammation.
Figure 4
Immunosurveillance, tumor-promoting and therapy-induced inflammation. A) Balance between immunosurveillance and tumor promoting inflammation in the tumor microenvironment. Tumor promoting cytokines act on immune and malignant cells to tilt the balance toward tumor promotion. Tumor promoting immunity dampens immunosurveillance, which otherwise inhibits tumor growth. B) Therapy-induced inflammation. Various forms of therapy induce death (necrosis) of malignant cells resulting in the release of necrotic products and damage-associated molecular patterns (DAMPs) that activate cytokine-producing inflammatory cells. These cytokines activate pro-survival genes in residual cancer cells, rendering them resistant to subsequent rounds of therapy. However, in some cases, therapy-induced inflammation augments the presentation of tumor antigens and stimulates an anti-tumor immune response that improves the therapeutic outcome.
Similar articles
- The role of the mediators of inflammation in cancer development.
Fernandes JV, Cobucci RN, Jatobá CA, Fernandes TA, de Azevedo JW, de Araújo JM. Fernandes JV, et al. Pathol Oncol Res. 2015 Jul;21(3):527-34. doi: 10.1007/s12253-015-9913-z. Epub 2015 Mar 5. Pathol Oncol Res. 2015. PMID: 25740073 Review. - Inflammatory signaling and cellular senescence.
Ren JL, Pan JS, Lu YP, Sun P, Han J. Ren JL, et al. Cell Signal. 2009 Mar;21(3):378-83. doi: 10.1016/j.cellsig.2008.10.011. Epub 2008 Oct 26. Cell Signal. 2009. PMID: 18992324 Free PMC article. Review. - Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms.
Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Elinav E, et al. Nat Rev Cancer. 2013 Nov;13(11):759-71. doi: 10.1038/nrc3611. Nat Rev Cancer. 2013. PMID: 24154716 Review. - Balancing the innate immune system in tumor development.
Hagerling C, Casbon AJ, Werb Z. Hagerling C, et al. Trends Cell Biol. 2015 Apr;25(4):214-20. doi: 10.1016/j.tcb.2014.11.001. Epub 2014 Nov 28. Trends Cell Biol. 2015. PMID: 25444276 Free PMC article. Review. - The inflammatory tumor microenvironment and its impact on cancer development.
de Visser KE, Coussens LM. de Visser KE, et al. Contrib Microbiol. 2006;13:118-137. doi: 10.1159/000092969. Contrib Microbiol. 2006. PMID: 16627962 Review.
Cited by
- A MR-PheWAS and bidirectional Mendelian randomization study: Exploring for causal relationships of pancreatic cancer.
Guan A, Li Z, Guo X. Guan A, et al. Medicine (Baltimore). 2024 Oct 11;103(41):e40047. doi: 10.1097/MD.0000000000040047. Medicine (Baltimore). 2024. PMID: 39465831 Free PMC article. - The Mechanism of APOBEC3B in Hepatitis B Virus Infection and HBV Related Hepatocellular Carcinoma Progression, Therapeutic and Prognostic Potential.
Yang X, Wang H, Yu C. Yang X, et al. Infect Drug Resist. 2024 Oct 17;17:4477-4486. doi: 10.2147/IDR.S484265. eCollection 2024. Infect Drug Resist. 2024. PMID: 39435460 Free PMC article. Review. - Prognostic and clinicopathological significance of C-reactive protein/albumin ratio (CAR) in patients with gastric cancer: A meta-analysis.
Yu J, Liu H, Zeng X, Zhao Y, Jiang D, Lu H, Qian J. Yu J, et al. PLoS One. 2021 Apr 26;16(4):e0250295. doi: 10.1371/journal.pone.0250295. eCollection 2021. PLoS One. 2021. PMID: 33901218 Free PMC article. Retracted. - A study of the correlation between M2 macrophages and lymph node metastasis of colorectal carcinoma.
Wang Y, Wang J, Yang C, Wang Y, Liu J, Shi Z, Chen Y, Feng Y, Ma X, Qiao S. Wang Y, et al. World J Surg Oncol. 2021 Mar 29;19(1):91. doi: 10.1186/s12957-021-02195-5. World J Surg Oncol. 2021. PMID: 33781288 Free PMC article. - A multi-omics-based investigation of the prognostic and immunological impact of necroptosis-related mRNA in patients with cervical squamous carcinoma and adenocarcinoma.
Zou J, Lin Z, Jiao W, Chen J, Lin L, Zhang F, Zhang X, Zhao J. Zou J, et al. Sci Rep. 2022 Oct 6;12(1):16773. doi: 10.1038/s41598-022-20566-0. Sci Rep. 2022. PMID: 36202899 Free PMC article. Clinical Trial.
References
- Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. - PubMed
- Angel P, Imagawa M, Chiu R, Stein B, Imbra RJ, Rahmsdorf HJ, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. - PubMed
- Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007a;220:47–59. - PubMed
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007b;13:1050–1059. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources