Endoplasmic reticulum stress and the inflammatory basis of metabolic disease - PubMed (original) (raw)
Review
Endoplasmic reticulum stress and the inflammatory basis of metabolic disease
Gökhan S Hotamisligil. Cell. 2010.
Abstract
The endoplasmic reticulum (ER) is the major site in the cell for protein folding and trafficking and is central to many cellular functions. Failure of the ER's adaptive capacity results in activation of the unfolded protein response (UPR), which intersects with many different inflammatory and stress signaling pathways. These pathways are also critical in chronic metabolic diseases such as obesity, insulin resistance, and type 2 diabetes. The ER and related signaling networks are emerging as a potential site for the intersection of inflammation and metabolic disease.
2010 Elsevier Inc. All rights reserved.
Figures
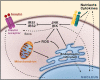
Figure 1. ER Stress and Inflammation
There are several potential avenues through which ER function is linked to inflammatory signaling. In response to ER stress, the three branches of the unfolded protein response (UPR) are activated. In the first branch, PERK-mediated attenuation of translation results in release of NF-κB from its inhibitor IκB. NF-κB moves to the nucleus and switches on expression of a variety of different genes involved in inflammatory pathways, such as those encoding the cytokines IL-1 and TNF-α. In the second branch of the UPR, activated IRE1α recruits tumor necrosis factor-α (TNF-α)-receptor-associated factor 2 (TRAF2), and this complex interacts with JNK and IκB kinase (IKK) and activates them. These inflammatory kinases then phosphorylate and activate downstream mediators of inflammation. The third branch of the UPR, the ATF6 pathway, also activates NF-κB. There is also crosstalk between the three branches. For example, spliced X-box binding protein 1 (XBP1s), as well as ATF4, induce production of the inflammatory cytokines IL-8, IL-6, and monocyte chemoattractant protein 1 (MCP1) by human endothelial cells. XBP1s has also been implicated in production of IFN-β when ER stress is combined with activation of Toll-like receptor (TLR) signaling and in IFN-α production by dendritic cells. The ability of XBP1s to expand the capacity of the ER for protein folding (and ER calcium stores) enables it to mediate calcium-dependent inflammatory responses in human bronchial epithelial cells, which produce IL-8. (Inset) Assembly of a putative metabolic inflammasome or metaflammasome. This protein complex integrates pathogen and nutrient sensing with ER stress, inflammatory kinases, insulin action, and metabolic homeostasis. The eIF2α kinase PKR (double-stranded RNA-activated protein kinase) is a core component of the metaflammasome and interacts directly with several inflammatory kinases such as IKK and JNK, insulin receptor signaling components such as IRS1, and the translational machinery via eIF2α. Nutrients, inflammatory mediators, and ER stress regulate PKR activity.
Figure 2. Insulin Signaling, Inflammation, and Stress Signals
In obesity, inflammatory mediators and lipids activate a signaling cascade that triggers inflammatory kinases such as JNK and IKK as well as protein kinase C, S6K, mTOR, and ERK. The activation of JNK and IKK results in the inhibition of insulin action in part through serine phosphorylation of insulin receptor substrates (IRS) 1 and 2. Energy or nutrient excess can trigger ER stress, which is directly linked to activation of inflammatory signaling pathways that then block insulin action and transcriptionally regulate production of inflammatory cytokines. Reactive oxygen species (ROS) that are produced during organelle stress and mitochondrial dysfunction also contribute to this cycle. The consequences are increased ER stress, increased inflammation, inhibition of insulin action, and possibly leptin action, culminating in systemic metabolic dysfunction.
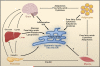
Figure 3. Crosstalk among Organs in Metabolic Regulation
Glucose derived from the diet or endogenous sources stimulates insulin secretion by the β cells of the pancreas. Insulin decreases glucose production by the liver, inhibits fat lipolysis, and increases glucose uptake by fat and muscle. Fat cells (adipocytes) release free fatty acids that increase gluconeogenesis and lipogenesis by the liver and reduce glucose uptake by muscle and insulin secretion by the pancreas. In addition, adipocytes secrete adipokines such as the proinflammatory cytokine TNF-α, leptin, and adiponectin that regulate food intake, energy expenditure, and insulin sensitivity either directly or via the brain. Adipocytes also secrete lipokines that modulate lipid metabolism in the liver and glucose metabolism in muscle. The brain monitors metabolite signals (such as serum glucose, free fatty acid levels) as well as hormones (insulin, leptin) from peripheral tissues resulting in the regulation of whole-body metabolism. The ER functions as a nutrient sensor. Obesity is associated with increased adiposity, chronic inflammation, and insulin resistance. Within the adipocyte, elevated lipid storage, lipogenesis, and adipokine synthesis may act as stress signals for the ER. In the liver, increased protein synthesis, lipogenesis, lipid transport, and gluconeogenesis influence ER function. An increased demand for insulin synthesis in pancreatic β cells may lead to disturbed ER homeostasis. An increase in circulating free fatty acids and inflammatory cytokines could also trigger ER stress in the hypothalamus through activation of IkB kinase (IKK). It is not clear how obesity-induced metabolic stress influences ER function in muscle.
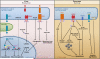
Figure 4. ER Stress, the UPR, and Metabolic Homeostasis
ER stress interferes with lipid metabolism, glucose homeostasis, and iron homeostasis in the liver (left panel). In response to ER stress, the cleaved form of ATF6 (ATF6-N) translocates to the nucleus and binds to SRE-bound SREBP2, which recruits the histone deacetylase HDAC1 that blocks SRE-mediated transcriptional activation. In obesity, ATF6-N can also bind to TORC2, decreasing the interaction of TORC2 with CREB and downregulating hepatic gluconeogenesis. Activated PERK phosphorylates eIF2α, which in turn increases production of C/EBPα and C/EBPβ proteins in the liver. These transcription factors induce expression of genes involved in lipid synthesis and gluconeogenesis. The spliced form of X-box binding protein 1 (XBP1s) can activate a subset of genes involved in lipogenesis. Overexpression of the chaperone Grp78 inhibits the ER stress-induced activation of SREBP1 and 2 and reduces hepatic steatosis in obese mice. ORP150 overexpression in the liver also reduces gluconeogenesis. The liver is also important for regulating iron homeostasis. ER stress may induce production of the iron-regulating peptide hormone hepcidin in hepatocytes, leading to abnormal iron homeostasis. The pancreatic β cell must maintain the proper balance between insulin synthesis, folding, and secretion, and the ER is intricately involved in this process (right panel). In early development, PERK is required for β cell formation and survival. IRE1 is required for maintenance of insulin production; eIF2α phosphorylation is necessary to halt insulin synthesis until folding demands have been met. The UPR-induced chaperone p58IPK is also necessary for β cell survival. Loss of the WFS1 gene, which encodes the ER transmembrane protein wolframin, results in increased ER stress and cell death. Unresolved ER stress in β cells may lead to apoptosis through IRE1-activated JNK phosphorylation and also through the downstream UPR mediator CHOP. CHOP downregulation during high insulin demand results in the survival and expansion of β cells and improved glucose homeostasis.
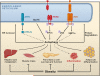
Figure 5. ER Stress, Autophagy, Inflammatory Signals, and Metabolism
The unfolded protein response (UPR) has been implicated in ER stress-induced autophagy, thus implicating autophagy in ER homeostasis. Potential actions of autophagy in stress recovery could include degradation of misfolded proteins and the promotion of ER turnover. Autophagy may also be involved in lipid droplet formation in the liver, b cell survival and function, adipocyte differentiation, muscle mass regulation, and inflammatory responses, all of which are disturbed in obesity. A role for autophagy in insulin action is not known.
Similar articles
- Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases.
Engin F, Hotamisligil GS. Engin F, et al. Diabetes Obes Metab. 2010 Oct;12 Suppl 2:108-15. doi: 10.1111/j.1463-1326.2010.01282.x. Diabetes Obes Metab. 2010. PMID: 21029307 Review. - Thematic review series: Adipocyte Biology. Adipocyte stress: the endoplasmic reticulum and metabolic disease.
Gregor MF, Hotamisligil GS. Gregor MF, et al. J Lipid Res. 2007 Sep;48(9):1905-14. doi: 10.1194/jlr.R700007-JLR200. Epub 2007 May 9. J Lipid Res. 2007. PMID: 17699733 Review. - Inflammation and endoplasmic reticulum stress in obesity and diabetes.
Hotamisligil GS. Hotamisligil GS. Int J Obes (Lond). 2008 Dec;32 Suppl 7(Suppl 7):S52-4. doi: 10.1038/ijo.2008.238. Int J Obes (Lond). 2008. PMID: 19136991 Free PMC article. Review. - The Unfolded Protein Response: Detecting and Responding to Fluctuations in the Protein-Folding Capacity of the Endoplasmic Reticulum.
Karagöz GE, Acosta-Alvear D, Walter P. Karagöz GE, et al. Cold Spring Harb Perspect Biol. 2019 Sep 3;11(9):a033886. doi: 10.1101/cshperspect.a033886. Cold Spring Harb Perspect Biol. 2019. PMID: 30670466 Free PMC article. Review. - Mitochondrial stress: a bridge between mitochondrial dysfunction and metabolic diseases?
Hu F, Liu F. Hu F, et al. Cell Signal. 2011 Oct;23(10):1528-33. doi: 10.1016/j.cellsig.2011.05.008. Epub 2011 May 15. Cell Signal. 2011. PMID: 21616143 Free PMC article. Review.
Cited by
- Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles.
Yuan X, Wang J, Tang X, Li Y, Xia P, Gao X. Yuan X, et al. J Transl Med. 2015 Jan 27;13:24. doi: 10.1186/s12967-015-0383-6. J Transl Med. 2015. PMID: 25623289 Free PMC article. - Alternative transcripts and 3'UTR elements govern the incorporation of selenocysteine into selenoprotein S.
Bubenik JL, Miniard AC, Driscoll DM. Bubenik JL, et al. PLoS One. 2013 Apr 16;8(4):e62102. doi: 10.1371/journal.pone.0062102. Print 2013. PLoS One. 2013. PMID: 23614019 Free PMC article. - Redox stress unbalances the inflammatory cytokine network: role in autoinflammatory patients and healthy subjects.
Lavieri R, Rubartelli A, Carta S. Lavieri R, et al. J Leukoc Biol. 2016 Jan;99(1):79-86. doi: 10.1189/jlb.3MR0415-159R. Epub 2015 Jul 21. J Leukoc Biol. 2016. PMID: 26199031 Free PMC article. Review. - A possible involvement of endoplasmic reticulum stress in biliary epithelial autophagy and senescence in primary biliary cirrhosis.
Sasaki M, Yoshimura-Miyakoshi M, Sato Y, Nakanuma Y. Sasaki M, et al. J Gastroenterol. 2015 Sep;50(9):984-95. doi: 10.1007/s00535-014-1033-0. Epub 2015 Jan 1. J Gastroenterol. 2015. PMID: 25552342 - Angiogenesis and liver fibrosis.
Elpek GÖ. Elpek GÖ. World J Hepatol. 2015 Mar 27;7(3):377-91. doi: 10.4254/wjh.v7.i3.377. World J Hepatol. 2015. PMID: 25848465 Free PMC article. Review.
References
- Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell. 2007;27:53–66. - PubMed
- Alexaki VI, Notas G, Pelekanou V, Kampa M, Valkanou M, Theodoropoulos P, Stathopoulos EN, Tsapis A, Castanas E. Adipocytes as immune cells: differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J. Immunol. 2009;183:5948–5956. - PubMed
- Bailey D, O'Hare P. Transmembrane bZIP transcription factors in ER stress signaling and the unfolded protein response. Antioxid. Redox Signal. 2007;9:2305–2321. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R01 DK064360/DK/NIDDK NIH HHS/United States
- R01 DK064360-07/DK/NIDDK NIH HHS/United States
- P30 DK040561-14/DK/NIDDK NIH HHS/United States
- R01 DK052539/DK/NIDDK NIH HHS/United States
- R01 DK052539-12/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical