Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors - PubMed (original) (raw)
Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors
Jae-Il Kim et al. J Biol Chem. 2010.
Abstract
Transmissible spongiform encephalopathies (TSEs) are a group of neurodegenerative diseases that are associated with the conformational conversion of a normal prion protein, PrP(C), to a misfolded aggregated form, PrP(Sc). The protein-only hypothesis asserts that PrP(Sc) itself represents the infectious TSE agent. Although this model is supported by rapidly growing experimental data, unequivocal proof has been elusive. The protein misfolding cyclic amplification reactions have been recently shown to propagate prions using brain-derived or recombinant prion protein, but only in the presence of additional cofactors such as nucleic acids and lipids. Here, using a protein misfolding cyclic amplification variation, we show that prions causing transmissible spongiform encephalopathy in wild-type hamsters can be generated solely from highly purified, bacterially expressed recombinant hamster prion protein without any mammalian or synthetic cofactors (other than buffer salts and detergent). These findings provide strong support for the protein-only hypothesis of TSE diseases, as well as argue that cofactors such as nucleic acids, other polyanions, or lipids are non-obligatory for prion protein conversion to the infectious form.
Figures
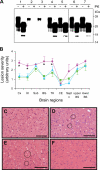
FIGURE 1.
Characterization of PrPSc and brain histopathology of rShaPrPPMCA-inoculated and control hamsters. A, Western blot analysis of PrPSc in brain homogenates from hamsters inoculated with PrP27–30 (lane 1), PrP27–30 seed after 10−14 dilution (lane 2), PrP27–30 seed subjected to mock PMCA (resulting in 10−14 dilution) in the absence of any rShaPrP substrate (lane 3), rShaPrP-(23–231)PMCA (lane 4), rShaPrP-(90–231)PMCA (lane 5), brain homogenate from rShaPrP-(23–231)PMCA-inoculated hamster (lane 6), and brain homogenate from rShaPrP-(90–231)PMCA-inoculated hamster (lane 7). Samples were analyzed before (−) and after (+) PK digestion (100 μg/ml, 1 h at 37 °C). The blot was probed with anti-PrP 3F4 antibody (1:10,000). B, lesion profiles in brains of hamsters inoculated with 263K scrapie PrP27–30 (red circle, n = 3), rShaPrP-(23–231)PMCA (blue triangle, n = 10), and rShaPrP-(90–231)PMCA (green square, n = 5). The profiles for hamsters inoculated with rShaPrP-(23–231)PMCA and rShaPrP-(90–231)PMCA were very similar to each other, but both were significantly different from that of hamsters inoculated with 263K scrapie PrP27–30. Specific brain regions showing statistically significant differences between hamsters inoculated with 263K scrapie PrP27–30 and rShaPrP-(90–231)PMCA are indicated by an asterisk (p < 0.001–0.04). For rShaPrP-(23–231)PMCA-inoculated hamsters, statistically significant differences (p < 0.001–0.02) from 263K-inoculated hamsters were observed in the same regions except for the thalamus. Symbols used for brain regions are: Cx, cerebral cortex; HI, hippocampus; Sub, subiculum; BG, basal ganglia; TH, thalamus; CE, cerebellum; Sept n, septal nuclei; BS, brainstem. Error bars indicate S.E. Statistical significance was determined using a two-tailed Student's t test. C–F, spongiform degeneration in basal ganglia of hamsters inoculated with 263K scrapie PrP27–30 (C), rShaPrP-(90–231)PMCA (sacrificed at 129 days after inoculation) (D), and brain homogenate of rShaPrP-(90–231)PMCA-infected hamsters (E). The histology of basal ganglia from control hamster inoculated with normal brain homogenate and sacrificed 368 days after inoculation is shown in panel F. Circles in panels D and E exemplify small clusters of typical vacuoles. Although 263K scrapie PrP27–30-inoculated hamsters showed widespread spongiform degeneration, the spongiform degeneration in rShaPrP-(90–231)PMCA-inoculated hamsters was much less severe, and this difference was maintained in the second passage. No spongiform degeneration was observed in control hamsters. Bar = 100 μm.
FIGURE 2.
Comparison of lesion profiles and PrPSc staining observed in first and second passage of rShaPrPPMCA prions. A, lesion profile in first (blue triangle, n = 10) and second (black open squares, n = 5) passage of rShaPrP-(23–231)PMCA; B, lesion profile in first (blue triangle, n = 5) and second (black open squares, n = 12) passage of rShaPrP-(90–231)PMCA. Lesion profiles for 263K scrapie-inoculated hamsters (red circle, n = 3) are shown for comparison. No statistically significant differences were found between profiles for first and second passages of both rShaPrP-(23–231)PMCA and rShaPrP-(90–231)PMCA (p > 0.05), but each of these profiles was significantly different from that for 263K scrapie (p < 0.00001–0.02). Symbols used for brain regions are: Cx, cerebral cortex; HI, hippocampus; Sub, subiculum; BG, basal ganglia; TH, thalamus; CE, cerebellum; Sept n, septal nuclei; BS, brainstem. Error bars indicate S.E. Statistical significance was determined using a two-tailed Student's t test. C–E, immunohistochemical staining of PrPSc in the white matter of corpus callosum (CC) and gray matter of cerebral cortex (Cor). C, hamsters inoculated with 263K scrapie PrP27–30; D, first passage of rShaPrP-(90–231)PMCA; E, second passage of rShaPrP-(90–231)PMCA. The square in panel C indicates perivascular deposits of PrPSc, and arrows indicate plaque-like deposits. Bar = 100 μm.
Similar articles
- Generation of genuine prion infectivity by serial PMCA.
Weber P, Giese A, Piening N, Mitteregger G, Thomzig A, Beekes M, Kretzschmar HA. Weber P, et al. Vet Microbiol. 2007 Aug 31;123(4):346-57. doi: 10.1016/j.vetmic.2007.04.004. Epub 2007 Apr 7. Vet Microbiol. 2007. PMID: 17493773 - In Vitro Approach To Identify Key Amino Acids in Low Susceptibility of Rabbit Prion Protein to Misfolding.
Eraña H, Fernández-Borges N, Elezgarai SR, Harrathi C, Charco JM, Chianini F, Dagleish MP, Ortega G, Millet Ó, Castilla J. Eraña H, et al. J Virol. 2017 Nov 30;91(24):e01543-17. doi: 10.1128/JVI.01543-17. Print 2017 Dec 15. J Virol. 2017. PMID: 28978705 Free PMC article. - PrP P102L and Nearby Lysine Mutations Promote Spontaneous In Vitro Formation of Transmissible Prions.
Kraus A, Raymond GJ, Race B, Campbell KJ, Hughson AG, Anson KJ, Raymond LD, Caughey B. Kraus A, et al. J Virol. 2017 Oct 13;91(21):e01276-17. doi: 10.1128/JVI.01276-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28835493 Free PMC article. - Prion diseases and their biochemical mechanisms.
Cobb NJ, Surewicz WK. Cobb NJ, et al. Biochemistry. 2009 Mar 31;48(12):2574-85. doi: 10.1021/bi900108v. Biochemistry. 2009. PMID: 19239250 Free PMC article. Review. - Prion encephalopathies of animals and humans.
Prusiner SB. Prusiner SB. Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
Cited by
- Recombinant Mammalian Prions: The "Correctly" Misfolded Prion Protein Conformers.
Ma J, Zhang J, Yan R. Ma J, et al. Viruses. 2022 Aug 31;14(9):1940. doi: 10.3390/v14091940. Viruses. 2022. PMID: 36146746 Free PMC article. Review. - Intraperitoneal Infection of Wild-Type Mice with Synthetically Generated Mammalian Prion.
Wang X, McGovern G, Zhang Y, Wang F, Zha L, Jeffrey M, Ma J. Wang X, et al. PLoS Pathog. 2015 Jul 2;11(7):e1004958. doi: 10.1371/journal.ppat.1004958. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26136122 Free PMC article. - Phosphatidylethanolamine as a prion cofactor: potential implications for disease pathogenesis.
Supattapone S. Supattapone S. Prion. 2012 Nov-Dec;6(5):417-9. doi: 10.4161/pri.21826. Epub 2012 Aug 16. Prion. 2012. PMID: 22895101 Free PMC article. Review. - Kosmotropic anions promote conversion of recombinant prion protein into a PrPSc-like misfolded form.
Diaz-Espinoza R, Mukherjee A, Soto C. Diaz-Espinoza R, et al. PLoS One. 2012;7(2):e31678. doi: 10.1371/journal.pone.0031678. Epub 2012 Feb 9. PLoS One. 2012. PMID: 22347503 Free PMC article. - Development of a new largely scalable in vitro prion propagation method for the production of infectious recombinant prions for high resolution structural studies.
Eraña H, Charco JM, Di Bari MA, Díaz-Domínguez CM, López-Moreno R, Vidal E, González-Miranda E, Pérez-Castro MA, García-Martínez S, Bravo S, Fernández-Borges N, Geijo M, D'Agostino C, Garrido J, Bian J, König A, Uluca-Yazgi B, Sabate R, Khaychuk V, Vanni I, Telling GC, Heise H, Nonno R, Requena JR, Castilla J. Eraña H, et al. PLoS Pathog. 2019 Oct 23;15(10):e1008117. doi: 10.1371/journal.ppat.1008117. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31644574 Free PMC article.
References
- Collinge J. (2001) Annu. Rev. Neurosci. 24, 519–550 - PubMed
- Aguzzi A., Polymenidou M. (2004) Cell 116, 313–327 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG14359/AG/NIA NIH HHS/United States
- NS44158/NS/NINDS NIH HHS/United States
- P01 AG014359/AG/NIA NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 NS052319/NS/NINDS NIH HHS/United States
- R01 NS044158/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials